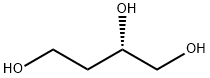
What is (S)-1,2,4-Butanetriol?
Feb 11,2020
(S)-(-)-1,2,4-Butanetriol can be prepared via reduction of (S)-malic acid in the presence of borane-dimethyl sulfide. (S)-(-)-1,2,4-Butanetriol may be used as a starting material in the enantioselective total syntheses of (+)-azimine and (+)-carpaine. It can also be used to prepare the organic building blocks (+)-3,4-epoxy-1-butanol, (2S,4S)-4-(hydroxymethyl)-2-ferrocenyl-1,3-dioxan, (S)-1,2,4-triacetoxybutane via acetylation with acetic anhydride, and (S)-1,2,4-tris-(3,5-dinitrobenzoy1oxy)butane via esterification with 3,5-dinitrobenzoyl chloride, and so on.
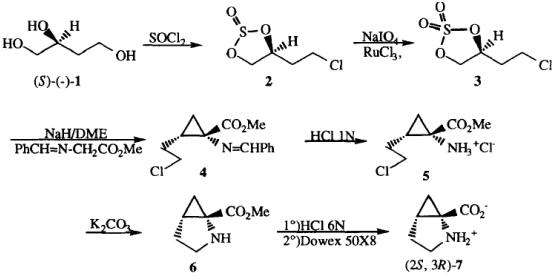
Hercouet et al. reported [1] its application on the synthesis of (-)- (2S, 3R)-Methanoproline. (S)-(-)-butanetriol 1 was converted to chlorosulfite 2 which was purified by flash chromatography. The following oxidation, using the Sharpless procedure, gave sulfate 3. Condensation of this sulfate on methyl benzylideneglycinate, at room temperature in DME in the presence of two equivalents of sodium hydride, was achieved following the procedure recently reported, to give the alkylated imine 4 in quantitative yield. This reaction is diastereospecific, only the Z isomer is obtained. Hydrolysis of the amino protective group by 1 N HCI gave the hydrochloride 510 in 80% yield, that cyclized to the desired aminoester 6 when treated with K2CO3. Hydrolysis of the ester group was realized by refluxing in 6 N HCI. The aminoacid zwitterion 7 was then quantitatively obtained using Dowex 50X8. This new synthesis can be perform on a multigram scale with 43% overall yield from 2 and without purification of intermediates.
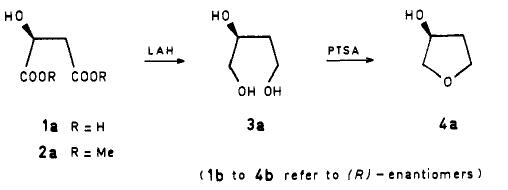
Tandon et al. reported [2] its application on the synthesis of (S)-( + )-3-Hydroxytetrahydrofuran. The procedure used for the first step (i.e., the LiAlH4 reduction of 2a) was improved to yield 72% of (S)-(-)-l,2,4-butanetriol (3a; reported yield 50%). The cyclodehydration step was carried out by heating 3a (neat) with a catalytic quantity of p-toluenesulfonic acid.
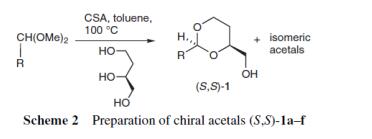
Geisler et al. reported [3] its application on the synthesis of chiral auxiliary for the preparation of (ps)-1-(diphenylphosphino)-2-formyl-1’,2’,3’,4’,5’- pentamethylferrocene. (3S)-4-Methoxybutane-1,3-diol [(S)-4], an important auxiliary for the synthesis of planar-chiral metallocenes, has been obtained from (S)-1,2,4-butanetriol via formation of an isomerically pure acetal of p-nitrobenzaldehyde, O-methylation and hydrolysis.
References
1.Hercouet A. et al. First Asymmetric Synthesis of (-)- (2S, 3R)-Methanoproline[J]. Tetrahedron: Asymmetry, 1996, 7(5):1267-1268.
2.Tandon VK. et al. Synthesis of Enantiomerically Pure (S)-( + )-3-Hydroxytetrahydrofuran and Its R Enantiomer from Malic or Tartaric Acid[J]. J. Org. Chem. 1983, 48:2767-2769
3.Geisler FM, Helmchen G. A Straightforward Synthesis of (3S)-4-Methoxybutane-1,3-diol and its Use as
Chiral Auxiliary for the Preparation of (pS)-1-(Diphenylphosphino)-2-formyl-1’,2’,3’,4’,5’-pentamethylferrocene[J]. Synthesis, 2006, 13:2201–2205x
);
You may like
Lastest Price from (S)-1,2,4-Butanetriol manufacturers
(S)-1,2,4-Butanetriol
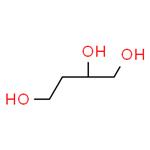
US $61.00-519.00/g2024-02-23
- CAS:
- 42890-76-6
- Min. Order:
- 5g
- Purity:
- 0.97
- Supply Ability:
- 25kg
(S)-1,2,4-Butanetriol
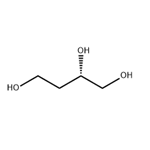
US $0.00-0.00/kg2022-09-20
- CAS:
- 42890-76-6
- Min. Order:
- 1kg
- Purity:
- 98%
- Supply Ability:
- 1Ton