What is the Charge of a Chlorine Ion?
According to the octet rule, atoms ought to have 8 electrons in their valence (outermost) electron shell to become stable. However, the atoms of most elements do not have eight valence electrons. They may gain or lose electrons to achieve an octet and become electrically charged, and are then called ions. Atoms that require only one or two electrons to complete their valence shells can acquire them and become negatively charged, as the electrons they now possess are more than the protons in their nucleus. These negatively charged ions are called anions. Other atoms have only a few valence electrons (3 or lower). The atoms lose these electrons, leaving their previously penultimate electron shell, which is full, to act as the valence shell. They then acquire a positive charge as the number of protons exceeds the number of electrons. These ions are called cations. In general, metals become cations, while non-metals become anions.
Chlorine is in Group 7 of the periodic table. Therefore, a chlorine atom has 7 electrons in its outer shell. The electronic configuration of Chlorine is as follows: Cl: 1s2 2s2 2p6 3s2 3p5
Here, the valence shell is a single electron short of an octet. To achieve it, the atom has to gain an electron.
Cl + e– → Cl–
The electronic configuration now is Cl: 1s22s22p63s23p6
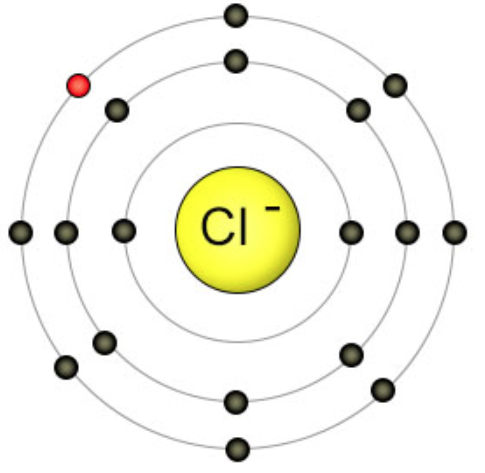
Cl– is called chloride and is an anion. It has a charge of -1 as the ion now has one electron more than its protons. In table salt, this electron comes from the sodium atom. When Chlorine gains an electron, its third energy level becomes full. An additional electron cannot join because it would need to come in at the fourth energy level. This far from the nucleus, the electron would not feel enough attraction from the protons to be stable. The positive sodium and negative chloride ions attract each other and form an ionic bond. The ions are more stable when they are bonded than they were as individual atoms.
In addition, The Group 7A elements have seven valence electrons in their highest-energy orbitals (ns2np5). This is one electron away from having a full octet of eight electrons, so these elements tend to form anions having -1 charges, known as halides: fluoride, F-; chloride, Cl-, bromide, Br-, and iodide, I-.
The chloride ion is an anion (negatively charged ion) with the charge Cl−. Chloride salts such as sodium chloride are often soluble in water. Other examples of ionic chlorides are calcium chloride CaCl2 and ammonium chloride NH4Cl. The chloride is also a neutral chlorine atom covalently bonded by a single bond to the rest of the molecule. For example, methyl chloride CH3Cl is an organic compound with a covalent C−Cl bond in which the Chlorine is not an anion. Other examples of covalent chlorides are carbon tetrachloride CCl4, sulfuryl chloride SO2Cl2, and monochloramine NH2Cl.
);