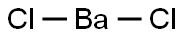Why does Barium Chloride burn green?
Barium Chloride, with the chemical formula BaCl2, is an inorganic compound commonly used in various industries and laboratory settings. It is a water-soluble salt of barium and has toxic properties. When burned, it produces a bright green color in flames. It is also known to be hygroscopic, meaning it absorbs moisture from the air.

Why does Barium Chloride burn green?
The heat of the flame excites the metals ions , causing them to emit visible light. The characteristics emission spectra is used to differentiate between the elements. The emission spectra of barium contains the wavelength corresponding to green colour ,hence barium gives green colour.
In wastewater treatment, Barium Chloride Dihydrate is employed. Additionally, it is used in the production of PVC stabilizers, barium chromate, barium fluoride, and oil lubricants. Due to its affordability and solubility, it finds extensive use in laboratories as a reagent for testing sulfate ions.
Industrially, Barium Chloride is primarily utilized for the purification of brine solutions in caustic chlorine plants. It is also involved in the production of heat treatment salts, steel case hardening, pigment manufacturing, and other barium salt production. When included in fireworks, it produces a vibrant green color. However, its toxic nature limits its use in certain applications.
What is flame test?
The flame test is a fast method for detecting certain elements in a sample. Although it is considered outdated and not entirely reliable, it used to be an important part of qualitative inorganic analysis. This technique is connected to both pyrotechnics and atomic emission spectroscopy. The colors produced by the flames can be explained by the principles of atomic electron transition and photoemission. Each element has specific energy levels (photons) required for electron transitions, resulting in unique flame colors.
Flame tests involve the thermal excitation of ions, which causes them to enter an energized state. These excited states later return to their ground state by releasing a photon. The energy of the excited state(s) and the corresponding emitted photon is specific to each element. The characteristics of the excited and ground states are solely determined by the element and are unaffected by any bonds. Consequently, molecular orbital theory is not relevant in this context. The emission spectrum observed during flame tests serves as the foundation for flame emission spectroscopy, atomic emission spectroscopy, and flame photometry.
);You may like
Related articles And Qustion
Lastest Price from Barium chloride manufacturers

US $610.00/T2024-04-09
- CAS:
- 10361-37-2
- Min. Order:
- 1T
- Purity:
- 99%
- Supply Ability:
- 6000T

US $0.00/KG2023-09-04
- CAS:
- 10361-37-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month