Zirconium hydride:Structure, Production and Uses
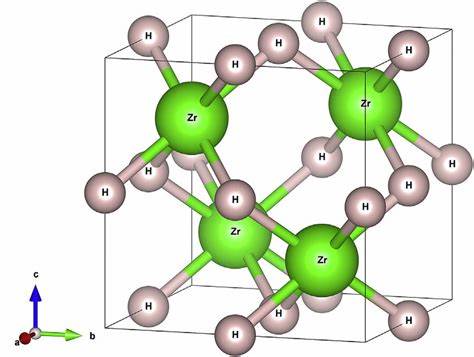
At 300℃, pure zirconium metal reversibly absorbs hydrogen until the stoichiometric dihydride ZrH2.00 (ε-phase) is formed. This compound crystallizes in a body-centered tetragonal structure. The reaction takes place in several stages, forming the metastable g-phase (ZrH), then the stable d-phase (ZrH1.59-1.67), and finally the e-phase with the highest hydrogen content (ZrH1.67-2.00). The eutectic is at 550℃ and 33 atom% H.
Zirconium hydride is thermally more stable than titanium hydride. At atmospheric pressure, it dissociates at 500℃. At 600 C it has lost ca. 6 % of its theoretical hydrogen content, at 800℃ 19 %, and at 900℃ 50 %. Hydrogen-free zirconium powder is produced by the dehydrogenation of powdered ZrH2 in vacuum (103 Pa) at 800℃.
Commercial supply
The chemical properties of zirconium hydride and titanium hydride are very similar, although zirconium hydride is much more sensitive towards oxidizing agents. It is inert to air and water at room temperature. The usual commercial product, a grayish black powder with particle size 2 – 6 mm, ignites in air at ca. 250 – 300℃. The hydride should therefore only be ground in an inert gas atmosphere (argon) or when wetted with water.
Production
Zirconium hydride that conforms to various quality requirements is produced either by direct hydrogenation of metallic zirconium or by the reduction of zirconium dioxide with calcium hydride, calcium, or magnesium in a hydrogen atmosphere (Degussa, Ventron). Zirconium sponge is hydrogenated at 300℃ mainly for the production of the pure hydride with a low hafnium content for the nuclear industry (96.9 – 97.9 % zirconium, 1.9 – 2.1 % hydrogen,<0.01 % hafnium, ≤0.14 % other metals), and also for the production of zirconium metal powder for the powder metallurgical industry.
Uses
Zirconium hydride is used in the nuclear industry as a moderator for thermal neutrons, especially in light water reactors and fast breeder reactors. It can be formed by warm pressing and is characterized by low neutron absorption and high thermal stability and mechanical strength. Very effective shielding materials contain a combination of a neutron absorber (e.g., boron carbide or zirconium) with a moderator such as zirconium hydride.
Zirconium hydride is also widely used as a getter in vacuum and ultra-high vacuum technology, e. g., for pipework and electric lights; it is able to bind up to 40 % oxygen or up to 20 % nitrogen.
Like titanium hydride, zirconium hydride can provide a bond between nonmetallic materials (e.g., SiN and SiC) and metals. Zirconium hydride is added at a concentration of 0.01 – 5 % to a matrix of silver, copper, or nickel alloys to form reactive brazing compounds.
);