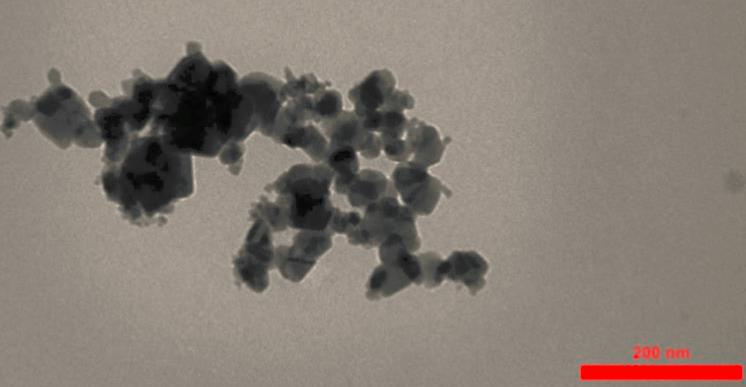
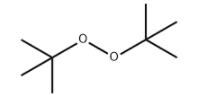
Oxides and peroxides
More
Less
An oxide is a chemical compound that contains at least one oxygen atom and one other element in its chemical formula. Peroxides are a group of compounds with the structure R−O−O−R. The O−O group in a peroxide is called the peroxide group or peroxo group. In contrast to oxide ions, the oxygen atoms in the peroxide ion have an oxidation state of −1.