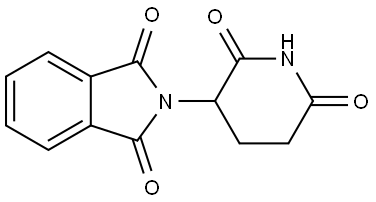
General Description
Needles or white powder.
Reactivity Profile
Organic amides/imides, such as THALIDOMIDE(50-35-1), react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Air & Water Reactions
Insoluble in water.
Fire Hazard
Flash point data for this chemical are not available; however, THALIDOMIDE is probably combustible.
Description
Thalidomide is a glutamic acid derivative first synthesized in
1953 by Swiss Pharmaceuticals; however, due to lack of pharmacological
effects, the development was discontinued. In
1954, Chemie Grünenthal, a German company, undertook the
development of thalidomide and, in 1957, thalidomide was
marketed as an anticonvulsant for the treatment of epilepsy.
Since the drug caused drowsiness, it was also marketed as
a sedative. Thalidomide was considered a safe and effective
drug that caused deep sleep with no hangover, and by the end
of the 1950s, 14 pharmaceutical firms were marketing the drug
in countries of Europe, Asia, Australia, the Americas, and Africa.
However, the drug was never approved for use in the United
States due to concerns about the safety of the drug raised by
Frances Kelsey, MD, a drug reviewer at Food and Drug
Administration (FDA). The approval process was delayed due
to her repeated requests for additional safety information from
William S. Merrell Company, the licensee of Chemie Grünenthal
that applied to market thalidomide in the United States.
Dr Kelsey’s concerns were mostly related to thalidomideinduced
neuropathy. Previous research had shown that drugs
that irritated nerves in adult rabbits could have adverse effects
on growth and cause deformities in fetal rabbits. During this
time, the use of the drug became widespread and, because it
was effective in alleviating morning sickness, it became popular
among pregnant women. In 1961, two physicians, William G.
McBride, MD of Australia and Widulind Lenz, MD of Germany,
associated the increase in malformation of the limbs (phocomelia)
and other congenital abnormalities with the use of
thalidomide by pregnant women. By late 1961, birth defects in
more than 12 000 children were associated with thalidomide
use, which forced companies to withdraw the drug worldwide.
The birth defects were due to thalidomide teratogenecity:
mainly phocomelia and malformation of ears, often accompanied
by malformation of the internal organ. In 1965, an
experimental use of thalidomide in patients with lepromatous
leprosy proved to be effective in treating painful skin lesions
that resulted from the inflammatory complications of leprosy.
In fact, experimental use of thalidomide had been extended to
a variety of diseases with various degrees of success, including
refractory rheumatoid arthritis, Crohn’s disease, human
immunodeficiency virus (HIV)-1 associated Kaposi’s sarcoma,
cutaneous lupus, prostate cancer, and colorectal cancer. In
1998, the FDA approved Thalomid as a therapy for erythema
nodosum leprosum (ENL), or leprosy. Subsequently in 2006,
Thalomid in combination with dexamethasone was approved
for treatment of multiple myeloma.
Originator
Contergan,Grunenthal,Germany
Definition
ChEBI: A dicarboximide that is isoindole-1,3(2H)-dione in which the hydrogen attached to the nitrogen is substituted by a 2,6-dioxopiperidin-3-yl group.
Indications
Thalidomide (Thalomid) is a derivative of glutamic acid
that is chemically related to glutethimide. It exerts a
number of biological effects as an immunosuppressive,
antiinflammatory, and antiangiogenic agent, yet its
mechanisms of action have not been fully elucidated.
Thalidomide potently inhibits production of tumor
necrosis factor (TNF) and interleukin (IL) 12, and its
effect on these and other cytokines may account for
some of its clinical effects.
Manufacturing Process
26 g of N-phthalyl glutaminic acid anhydride are melted with 12 g of urea in
an oil bath at 170-180°C until the reaction is completed, which takes about 20
min. The reaction takes place with violent evolution of carbon dioxide and
ammonia. After cooling, the reaction product is recrystallised by fractionation
from 95% alcohol, and the first fraction may contain phthalic acid derivatives.
The required product N-(2,6-dioxo-3-piperidyl)-phthalimide melts at 269-
271°C. The yield is about 65-70% of the theoretical.
Brand name
Algosediv;Asidon;Bonbrain;Contergan;Distaval;Funed;Glutanon;Hippuzon;Imidan;Isomin;Kevadone;Nerufatin;Neurosedyn;Pantosedive;Pro-ban;Quetimid;Sanodormin;Sedalis;Sedoval;Shinaito;Shinnibrol;Sleepan;Softenil;Softenon;Talimol;Tlargan;Yodomin.
Therapeutic Function
Sedative, Hypnotic, Antiarthritic
World Health Organization (WHO)
Notwithstanding the highly potent teratogenic action of
thalidomide, this drug retains a place in the treatment of reactional lepromatous
leprosy and several serious dermatological conditions refractory to other
treatment. In many countries, the competent authorities have granted exemption
from licensing requirements to enable doctors to obtain limited supplies of
thalidomide under strictly controlled circumstances for use in named patients.
Arrangements have also been made by some national drug regulatory authorities
for thalidomide to be used in institutions concerned with the treatment of leprosy.
Health Hazard
Thalidomide is a strong teratogen. Exposureto this compound during the first trimesterof pregnancy resulted in deformities inbabies. Infants born suffered from ameliaor phocomelia, the absence or severe shortening of limbs. Administration of thalidomide in experimental animals caused fetaldeaths, postimplantation mortality, and specific developmental abnormalities in theeyes, ear, central nervous system, musculoskeletal system, and cardiovascular system. Several thousand children were affected.The drug has been withdrawn from themarket.Thalidomide is usually administeredorally. Its toxicity is dose dependent. Someother effects are drowsiness, constipation andrash and nerve damage in the arms and legs.Interest in thalidomide resurged in recentyears because of its antitumor activity in thetreatment of multiple myeloma (Oxberry andJohnson 2006). The compound is an inhibitorof angiogenesis, that is, it prevents formationof new blood vessels in tumors. Also, it hasbeen found to be effective in treating AIDS-related Kaposis sarcoma.
Biological Activity
Teratogen, sedative-hypnotic with inherent anti-inflammatory properties. A selective inhibitor of tumor necrosis factor α (TNF- α ) synthesis.
Biochem/physiol Actions
(±)-Thalidomide selectively inhibits biosynthesis of tumor necrosis factor α (TNF-α). It also functions as an inhibitor of angiogenesis, an immunosuppressive agent, a sedative and a teratogen. Furthermore, thalidomide is known to exhibit antitumor functions in refractory multiple myeloma.
Mechanism of action
Its absorption from the gastrointestinal tract is slow,
with peak plasma levels being reached after 3 to 6
hours. It appears to undergo nonenzymatic hydrolysis in
the plasma to a large number of metabolites.The elimination
half-life is approximately 9 hours.
Clinical Use
Thalidomide is approved for use in the United
States for the treatment of cutaneous manifestations
of erythema nodosum leprosum, a potentially lifethreatening
systemic vasculitis that occurs in some patients
with leprosy.Although not approved for other indications,
thalidomide has also been shown to be very
effective in the management of Beh?et’s disease, HIVrelated
mucosal ulceration (aphthosis), and select cases
of lupus erythematosus.
Drug interactions
Potentially hazardous interactions with other drugs
Thalidomide enhances the effects of barbiturates,
alcohol, chlorpromazine and reserpine.
Use with caution with other drugs that can cause
peripheral neuropathy.
Metabolism
Thalidomide is metabolised almost exclusively by nonenzymatic hydrolysis. In plasma, unchanged thalidomide
represents 80
% of the circulatory components.
Unchanged thalidomide was a minor component
(<3
% of the dose) in urine. In addition to thalidomide,
hydrolytic products N-(o-carboxybenzoyl) glutarimide
and phthaloyl isoglutamine formed via non-enzymatic
processes are also present in plasma and in urine.
Toxicity evaluation
The mechanism of teratogenecity of the thalidomide is not
clearly understood; however, recent studies have shed some light
on the potential mechanism. CRBN has been identified as
a thalidomide-binding protein. The teratogenic effects start with
binding of thalidomide to CRBN and inhibiting the associated
ubiquitin ligase activity.Many E3 ubiquitin ligases are important
for various physiological processes such as cell cycle regulation,
carcinogenesis, immune response, and development. It has been
shown that CRBN forms an E3 ubiquitin ligase complex with
damaged DNA binding protein 1 (DDB1) and Cullin-4A
(Cul4A), which are important factors for expression of the
fibroblast growth factor Fgf8 in zebra fish and chicks as well as
the limb outgrowth. In thalidomide-treated zebra fish embryos,
formation of proximal endoskeletal disc of the pectoral fin was
severely inhibited and the otic vesicle size was significantly
reduced. Pectoral fins and otic vesicles in fish share common
molecular pathways with tetrapod limbs and ears development.
Down-regulation of the CRBN complex causes similar developmental
defects in zebra fish. Thalidomide does not cause limb
malformations in rodents but does in rabbits, monkeys. In
pregnant rats, thalidomide can cause other types of developmental
defects such as vertebral column, rib, and eye malformation.
There is very strong conservation of the amino acid
sequence of CRBN between rat, rabbit, monkey, mouse and
human and has been shown that they can bind to thalidomide.
The role and importance of CRBN in different species have not
been identified; therefore, the degree of teratogenecity could
depend in part on the nature of the protein substrates that are
modified by CRBN activity during embryonic development.
There are some species differences in teratogenecity between
thalidomide and its close analogs.
References
1) Ito et al. (2010), Identification of a primary target of thalidomide teratogenicity; Science, 327 1345
2) Weglicki et al. (1993), Inhibition of tumor necrosis factor-alpha by thalidomide in magnesium deficiency; Mol. Cell. Biochem., 129 195
3) D’Amato et al. (1994), Thalidomide is an inhibitor of angiogenesis ; Proc. Natl. Acad. Sci. USA, 91 4082