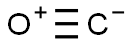Chemical Properties
Carbon monoxide (CO) is a colorless, odorless, tasteless gas that is extremely hazardous.
CO can be formed from incomplete burning of gasoline, wood, kerosene, or other fuels.
CO is also found in cigarette smoke and vehicle exhaust. In homes, CO can build up from a
poorly vented or malfunctioning heater, furnace, range, or any appliance that runs on natural gas or oil. The presence of CO is very common inside and outside workplaces, around
heaters: improper use of gas or kerosene-fi red heaters, gas-fi red central heating equipment combined with improper venting or chimney, due to blocked heating fl ues, improper
fl ue vent connector, or hood installation, inadequate combustion air, from exhaust, and
gas-fi red water heaters.
Uses
As reducing agent in metallurgical operations especially in the Mond process for the recovery of nickel; in organic synthesis especially in the Fischer-Tropsch processes for petroleum-type products and in the oxo reaction; in the manufacture of metal carbonyls.
General Description
A colorless cryogenic liquid. Prolonged exposure to carbon monoxide rich atmospheres may be fatal. Contact with the liquid can cause severe frostbite. Less dense than air. Easily ignited and a flame can flash back to the source of a leak very easily. Burns with a violet flame. Under prolonged exposure to fire or intense heat the containers may rupture violently and rocket. CARBON MONOXIDE, REFRIGERATED LIQUID (CRYOGENIC LIQUID)(630-08-0) is used in organic synthesis, metallurgy, and a fuel.
Reactivity Profile
Contact of very cold liquefied gas with water may result in vigorous or violent boiling and extremely rapid vaporization. If the water is hot, a liquid "superheat" explosion may occur. Pressures may build to dangerous levels if the liquid contacts water in a closed container [Handling Chemicals Safely 1980]. Reacts explosively with bromine trifluoride at high temperatures or concentrations [Mellor 2, Supp. 1:166 1956]. The same is true for various oxidizers such as: chlorine dioxide, oxygen (liquid), peroxodisulfuryl difluoride. Reacts with lithium to give lithium carbonyl, which detonates violently with water, igniting the gaseous products [Mellor 2, Supp 2:84 1961]. Potassium and sodium metals behave similarly. Cesium oxide, iron(III) oxide, and silver oxide all react, in the presence of moisture, at ambient temperatures with carbon monoxide causing ignition, [Mellor, 1941, vol. 2, 487].
Air & Water Reactions
Highly flammable.
Hazard
Highly flammable, dangerous fire and
explosion risk. Flammable limits in air 12–75% by
volume. Toxic by inhalation. Note: Carbon monox-
ide has an affinity for blood hemoglobin over 200
times that of oxygen. A major air pollutant.
Health Hazard
CO is a highly toxic gas often called a chemical asphyxiant. When inhaled, it combines
with hemoglobin more readily than oxygen does, displacing oxygen from hemoglobin,
thereby interfering with oxygen transport by the blood. The early symptoms of CO poisoning include, headaches, nausea, and fatigue, which are often mistaken for the fl u
because CO is not detected in the home. Prolonged exposure to CO causes deleterious
health effects, brain damage, and eventually death. CO poisoning can happen to anyone,
anytime, almost anywhere. CO poisoning is often confused with the fl u. Depending on
the period of exposure and concentration of CO, poisoning may be severe, moderate, or
mild. (i) Extreme exposures cause confusion, drowsiness, rapid breathing or pulse rate,
vision problems, chest pain, convulsions, seizures, loss of consciousness, cardiorespiratory failure, and death. (ii) Moderate exposures cause severe throbbing headache, drowsiness, confusion, vomiting, and fast heart rate. (iii) Mild exposures cause slight headache,
nausea, fatigue (often described as “fl u-like” symptoms).
The symptoms of CO poisoning include, but are not limited to, drowsiness, nausea,
tiredness, vomiting, headaches, dizziness, visual changes, abdominal pain, chest pains,
memory and walking problems, brain damage and, in severe cases, death. Exposure to
high concentrations of CO causes severe headache, weakness, dizziness, irregular heart
beat, seizures, coma, respiratory failure, and unconsciousness.
The toxicity of CO results from its very tight binding to hemoglobin, the species that
carries oxygen from the lungs to the bodily tissues. For hemoglobin to work, it can’t bind
oxygen very tightly (otherwise it couldn’t release it at its destination). Unfortunately, CO
Health Hazard
TOXIC; Extremely Hazardous. Inhalation extremely dangerous; may be fatal. Contact with gas or liquefied gas may cause burns, severe injury and/or frostbite. Odorless, will not be detected by sense of smell.
Potential Exposure
Carbon monoxide is used in metallurgy as a reducing agent, particularly in the Mond process
for nickel; in organic synthesis, especially in the
Fischer�Tropsch process for petroleum products, and in
the oxo reaction; and in the manufacture of metal carbonyls. It is usually encountered in industry as a waste product of incomplete combustion of carbonaceous material
(complete combustion produces CO2). The major source of
CO emission in the atmosphere is the gasoline-powered
internal combustion engine. Special industrial processes
which contribute significantly to CO emission are iron
foundries, particularly the cupola; fluid catalytic crackers;
fluid coking; and moving-bed catalytic crackers in thermal
operations in carbon black plants; beehive coke ovens;
basic oxygen furnaces, sintering of blast furnace feed in
steel mills; and formaldehyde manufacture. There are
numerous other operations in which a flame touches a surface that is cooler than the ignition temperature of the gaseous part of the flame where exposure to CO may occur,e.g., arc welding, automobile repair; traffic control; tunnel
construction; firefighting; mines, use of explosives, etc.
Fire Hazard
EXTREMELY FLAMMABLE. May be ignited by heat, sparks or flames. Flame may be invisible. Containers may explode when heated. Vapor explosion and poison hazard indoors, outdoors or in sewers. Vapors from liquefied gas are initially heavier than air and spread along ground. Vapors may travel to source of ignition and flash back. Runoff may create fire or explosion hazard.
First aid
Gas: Move victim to fresh air. Call emergency
medical care. Apply artificial respiration if victim is not
breathing. Do not use mouth-to-mouth method if victim
ingested or inhaled the substance; induce artificial respiration with the aid of a pocket mask equipped with a oneway valve or other proper respiratory medical device.
Administer oxygen if breathing is difficult. Remove and
isolate contaminated clothing and shoes. In case of contact
with substance, immediately flush skin or eyes with running water for at least 20 minutes. In case of contact with
liquefied gas, thaw frosted parts with lukewarm water.
Keep victim warm and quiet. Keep victim under observation for 24 to 48 hours. Effects of contact or inhalation may
be delayed. Ensure that medical personnel are aware of the
material(s) involved and take precautions to protect
themselves.
Refrigerated liquid: Move victims to fresh air. Call emergency medical care. Apply artificial respiration if victim is
not breathing. Administer oxygen if breathing is difficult.
Remove and isolate contaminated clothing and shoes. In
case of contact with substance, immediately flush skin or
eyes with running water for at least 20 minutes. In case of
contact with liquefied gas, thaw frosted parts with lukewarm water. Keep victim warm and quiet. Keep victim
under observation. Effects of contact or inhalation may be
delayed. Ensure that medical personnel are aware of the
material(s) involved and take precautions to protect
themselves.
Shipping
UN1016 Carbon monoxide, compressed,
Hazard class: 2.3; Labels: 2.3-Poisonous gas; 2.1-
Flammable gas, Inhalation Hazard Zone D. NA9202
Carbon monoxide, refrigerated liquid (cryogenic liquid),
Hazard class: 2.3; Labels: 2.3-Poisonous gas; 2.1-
Flammable gas, Domestic (United States), Inhalation
Hazard Zone D. Cylinders must be transported in a secureupright position, in a well-ventilated truck. Protect cylinder and labels from physical damage. The owner of the
compressed gas cylinder is the only entity allowed by federal law (49CFR) to transport and refill them. It is a violation of transportation regulations to refill compressed gas
cylinders without the express written permission of the
owner.
Incompatibilities
Forms extremely explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. In the presence of finely dispersed
metal powders the substance forms toxic and flammable
carbonyls. May react vigorously with oxygen, acetylene,
chlorine, fluorine, nitrous oxide.
Description
Carbon monoxide is a colorless, odorless, tasteless, flammable, toxic gas.Carbon monoxide is produced when carbon and carbon compounds undergo incomplete combustion. The inefficient combustion of carbon fuels for heating results in the production of carbon monoxide, which may result in high CO concentrations in indoor environments. The use of carbon fuel heaters without adequate ventilation can result in deadly conditions. Each year several hundred people in the United States die from CO poisoning, and 10,000 patients are treated in hospitals for CO exposure.Cars and other forms of transportation are a major source of carbon monoxide pollution in cities.
Waste Disposal
Return refillable compressed
gas cylinders to supplier. Dissolve or mix the material with
a combustible solvent and burn in a chemical incinerator
equipped with an afterburner and scrubber. All federal,
state, and local environmental regulations must be
observed. Carbon monoxide can also be recovered from
gas mixtures as an alternative to disposal.
Physical properties
Colorless, odorless and tasteless gas; density 1.229 g/L; very flammable,burns in air with a bright blue flame; liquefies at -191.5°C; solidifies at -205°C; critical temperature -140°C, critical pressure 34.53 atm, critical vol ume 93 cm3/mol; soluble in chloroform, acetic acid, ethyl acetate, ethanol, and ammonium hydroxide; sparingly soluble in water (2.3 mL/100 mL water at 20°C).
Occurrence
Carbon monoxide is found in varying concentrations in unventilated and confined spaces resulting from partial oxidation of carbonaceous matter. Burning wood, paper, kerosene, or other organic materials in inadequate air can produce this gas. It also is found in automobile exhaust and tobacco smoke emissions.
Carbon monoxide has many important industrial applications. It is used in Fischer-Tropsch process to produce liquid or gaseous hydrocarbons, synthet ic fuels and many oxygenated derivatives. This process was applied before and during World War II to produce synthetic fuels. Probably the most important application of this compound involves production of oxygenated organics in the Synthol process and in oxo synthesis. Many aliphatic alcohols, alehydes and ketones are produced by catalytic hydrogenation of carbon monoxide. Oxo synthesis produces aldehydes from olefins. Carbon monoxide also is the start ing material for preparing metal carbonyls. In metallurgy, it is used as a reducing agent to reduce oxides. In the Mond process it recovers nickel.
History
Carbon monoxide is a colorless, odorless, tasteless, flammable, toxic gas. It was first identified by the Spanish alchemist Arnold of Villanova (1235–1313), who noted the production of a poisonous gas when wood was burned. The formal discovery of carbon monoxide is credited to the French chemist Joseph Marie Fran?ois de Lassone (1717–1788) and the British chemist Joseph Priestley (1733–1804). The former prepared carbon monoxide by heating carbon in the presence of zinc, and for a time the compound was incorrectly identified as hydrogen. William Cumberland Cruikshank (1745–1800) correctly determined that carbon monoxide was an oxide of carbon in 1800.
Definition
A colorless flammable toxic gas
formed by the incomplete combustion of
carbon. In the laboratory it can be made by
dehydrating methanoic acid with concentrated
sulfuric acid:
HCOOH – H2O → CO
Industrially, it is produced by the oxidation
of carbon or of natural gas, or by the
water-gas reaction. It is a powerful reducing
agent and is used in metallurgy.
Carbon monoxide is neutral and only
sparingly soluble in water. It is not the anhydride
of methanoic acid, although under
extreme conditions it can react with
sodium hydroxide to form sodium
methanoate. It forms metal carbonyls with
transition metals, and its toxicity is due to
its ability to form a complex with hemoglobin.
Production Methods
Carbon monoxide is formed during combustion of carbonaceous
materials in oxygen (when carbon is in excess), or it
can be formed (with oxygen) by thermal decomposition
of carbon dioxide (>2000° °C). It can be generated by improperly vented cooking and heating appliances including
coal stoves, furnaces, and gas appliances when the oxygen
supply is insufficient. Other sources include exhaust of
internal combustion engines, structural fires, and tobacco
products. Carbon monoxide can also be formed endogenously
by normal heme turnover or during the metabolism
of selected hydrocarbons, such as methylene chloride. Not
surprisingly, CO is one of the most common agents of
inadvertent human intoxication in both occupational and
nonoccupational environments.
Reactions
Chemically, carbon monoxide is (1) reactive with oxygen to form CO2 accompanied by a transparent blue flame and the evolution of heat, but the fuel value is low (320 Btu per ft3), (2) reactive with chlorine, forming carbonyl chloride COCl2 in the presence of light and a catalyzer, (3) reactive with sulfur vapor at a red heat, forming carbonyl sulfide COS, (4) reactive with hydrogen, forming methyl alcohol, CH3OH or methane CH4 in the presence of a catalyzer, (5) reactive with nickel (also iron, cobalt, molybdenum, ruthenium, rhodium, osmium, and iridium) to form nickel carbonyl, Ni(CO)4 (and carbonyls of the other metals named), (6) reactive with fused NaOH, forming sodium formate, HCOONa, (7) reactive with cuprous salt dissolved in either ammonia solution or concentrated HCl, which solutions are utilized in the estimation of carbon monoxide in mixtures of gases, e.g., flue gases of combustion, coal gas, exhaust gases of internal combustion engines, (8) reactive with iodine pentoxide at 150 °C. For the reaction of carbon monoxide with oxygen to form CO2 finely divided iron or palladium wire is used as a catalyzer; for the reaction of carbon monoxide with H2O vapor to form CO2 plus hydrogen (“water gas reaction”) important studies have been made of the conditions; and for the reaction of CO2 plus carbon (hot) similar important studies have been made (at 675 °C, 50% CO2 plus 50% CO; at 900°C, 5% CO2 plus 95% CO). The reaction of carbon plus oxygen at such a temperature as produces carbon monoxide (say 900 °C, 95% CO plus 5% CO2) and evolves heat; while the reaction of carbon plus CO2, producing carbon monoxide at the same temperature absorbs heat. Accordingly it is possible to arrange the oxygen (free or as air) and CO2 supply ratio in such a way that the desired temperature may be continuously maintained. The reduction of CO2 by iron forms carbon monoxide plus ferrous oxide.
Purification Methods
Iron carbonyl is a likely impurity in CO stored under pressure in steel tanks. It can be decomposed by passing the gas through a hot porcelain tube at 350-400o. Passage through alkaline pyrogallol solution removes oxygen (and CO2). Removal of CO2 and water are effected by passage through soda-lime followed by Mg(ClO4)2 or P2O5 and collected over Hg. Carbon monoxide can be condensed and distilled at -195o. It is sparingly soluble in H2O but is readily absorbed by a solution of CuCl in HCl to give the white crystalline adduct CuCl.CO.2H2O. It burns in air with a bright blue flame but a mixture of 2volumes of CO and 1volume of O2 explode when kindled, although in a small jar the combustion is not violent. HIGHLY POISONOUS gas as it reacts with haemoglobin to form bright red carboxyhaemoglobin which is stable and not readily decomposed by oxygen. [Gilliland & Blanchard Inorg Synth II 81 1946, Glemser in Handbook of Preparative Inorganic Chemistry (Ed. Brauer) Academic Press Vol I p 645-646 1963.]
Flammability and Explosibility
Carbon monoxide is a flammable gas. It forms explosive mixtures with air in the
range of 12.5 to 74% by volume.
Industrial uses
Carbon monoxide (CO) is a product of incompletecombustion, and is very reactive. It is oneof the desirable products in synthesis gas formaking chemicals; the synthesis gas made fromcoal contains at least 37% CO. It is also recoveredfrom top-blown O2 furnaces in steel mills.It reacts with H2 to form methanol, which is then catalyzed by zeolites into gasoline. Aceticacid is made by methanol carbonylation, andacrylic acid results from the reaction of CO,acetylene, and methanol.
Materials Uses
Steel and other common metals are satisfactory
for use with dry, sulfur-free carbon monoxide at
pressures up to 2000 psig (13 790 kPa). Iron,
nickel, and other metals can react with carbon
monoxide at elevated pressures to form carbonyls
in small quantities. The presence of moisture
and sulfur-containing impurities in carbon monoxide
appreciably increases its corrosive action
on steel at any pressure. High-pressure plant
equipment is often lined with copper for increased
resistance to carbon monoxide attack.
Very highly alloyed chrome steels are sufficiently
resistant to corrosion by carbon monoxide
containing small amounts of sulfur-bearing
impurities. Users are strongly urged to make
stress corrosion tests of samples of proposed
construction materials in order to select one that
will withstand the high-pressure use of carbon
monoxide under actual conditions.
Physiological effects
Carbon monoxide is a chemical asphyxiant and
acts toxically by combining with the hemoglobin
of the red blood cells to form the stable
compound carbon monoxide-hemoglobin. It
thus prevents the hemoglobin from taking up oxygen, thereby depriving the body of the oxygen
needed for metabolic respiration. The affinity
of carbon monoxide for hemoglobin is about
300 times the affinity of oxygen for hemoglobin.
The inhalation of concentrations as low as
0.04 percent will result in headache and discomfort
within 2 to 3 hours. Inhalation of a 0.4 percent
concentration in air proves fatal in less than
I hour. Lacking odor and color, carbon monoxide
gives no warning of its presence, and inhalation
of heavy concentrations can cause sudden
unexpected collapse.
Environmental Fate
CO has varied effects on multiple enzymatic reactions and
processes. Most easily seen and measured via co-oximetry is its
high affinity and binding to Hb. This results in an overall lack
of oxygen carrying capacity along with a shift of the oxygen
dissociation curve to the left so that even available oxyhemoglobin
is less able to offload oxygen to tissue sites. This,coupled with CO’s ability to bind to and arrest cellular
metabolism, results in global hypoxemia. The overall lack of
tissue perfusion and energy production results in metabolic
lactic acidosis.
CO also has the ability to bind to other globins, most
importantly myoglobin. Significant myoglobin binding
results in lack of tissue oxygenation to heart and myocardial
damage.
The final high-risk organ system affected after CO exposure
is the central nervous system. CO has the ability to cause
delayed neuropsychiatric sequelae in addition to the acute
effects seen as a result of hypoxemia. This is thought to be due
to delayed lipid peroxidation achieved through the displacement
of nitric oxide. A reperfusion-like injury occurs in these
cases.
storage
cylinders of carbon monoxide
should be stored and used in a continuously ventilated gas cabinet or fume hood.
Local fire codes should be reviewed for limitations on quantity and storage
requirements.
Toxicity evaluation
Exposure to this colorless, odorless gas occurs primarily though
inhalation. CO exposure associated with the paint stripper
methylene chloride is unique in that methylene chloride is biologically
metabolized toCOin vivo. Dermal, oral, and inhalation
exposure to methylene chloride can cause CO poisoning.
Aside from tobacco smoke, the most important sources of
CO exposure for most individuals are the emissions created by
internal combustion engines of vehicles and in household and
occupational locations where combustion occurs. Specific sources
of exposure include the burning of wood, charcoal, natural
gas, or propane for heating and cooking, and propane-powered
indoor equipment such as forklifts and ice rink resurfacers.
Average levels of CO in homes without gas stoves vary from
0.5 to 5 ppm. Levels near properly adjusted gas stoves are often
5–15 ppm, and those near poorly adjusted stoves may be
30 ppm or higher. CO exposures occur in a variety of occupational
settings. The number of persons occupationally exposed
to CO in the working environment is greater than for any other
physical or chemical agent. The smoke of a cigarette contains
approximately 14 mg of CO. The smoke of cigars ranges from
approximately 38 mg for little cigars to almost 100 mg for large
and premium cigars. CO in secondhand tobacco smoke has led
to levels of CO as high as 50 ppm.
GRADES AVAILABLE
Carbon monoxide is available for commercial
and industrial use in various grades of purity
ranging from a minimum 98 percent to 99.99
percent.