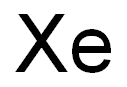Chemical Properties
Colorless, odorless gas or liquid. Gas
(at STP) has d 5.8971 g/L (air = 1.29 g/L), dielectric
constant 1.0012 (25C) (1 atm); liquid has bp
?108.12C (1 mm Hg), d (at bp) of 1.987 g/cc; liquefaction
temp?106.9C.Chemically unreactive but
not completely inert. Noncombustible.
Description
Krypton, neon, and xenon are rare atmos�pheric gases. Each is odorless, colorless, taste�less, nontoxic, monatomic, and chemically inert.
All three together constitute less than 0.002
percent of the atmosphere with approximate
concentrations in the atmosphere of 18 ppm for
neon, 1.1 ppm for krypton, and 0.09 ppm for
xenon. Few users of the three gases need them
in bulk quantities, and the three are shipped
most often in single cylinders and glass liter
flasks.
Radon, a radioactive rare gas, is not treated in
this book because it has little or no practical
application at present. It is the heaviest gas
known (density at 70°F and 1 atm, 0.61 Ib/ft3; at
21.1°C and 1 atm, 9.8 kg/m3.
Among the rare gases, neon, krypton, and xe�non in particular ionize at lower voltages than
other gases, and the brilliant, distinctive light
they emit while conducting electricity in the
ionized state accounts for one of their primary
uses. Their characteristic colors as ionized con�ductors are red for neon, yellow-green for
krypton, and blue to green for xenon. Similarly,
argon and helium are also used for this purpose
and emit red or blue for argon and yellow for
helium. These latter two gases are treated in
separate monographs.
Physical properties
Xenon has a relatively high atomic weight and is about 4.5 times heavier than air. It is colorless,tasteless, and odorless. Its critical temperature is comparatively high at 16.6°C, which isfar above oxygen (–188°C). This means that xenon will boil away from commercial fractionaldistillation of liquid oxygen.
Xenon’s melting point is –111.79°C, its boiling point is –108.12°C, and its density is0.005887g/cm3.
Isotopes
There are 46 isotopes of xenon. Nine of these are stable. Two of the stableisotopes are radioactive, but with half-lives long enough to be considered stable.They are Xe-124 (1.1×10+17years) and Xe-136 (3.6×10+20 years). The 47 manmadeartificial radioactive isotopes have half-lives ranging from 150 nanoseconds to11.9 days.
Origin of Name
The word “xenon” is derived from the Greek word xenon, meaning
“stranger.”
Occurrence
Xenon is found in trace amounts in the atmosphere. It makes up just 0.086 ppm by volumeof air. Xenon is the rarest of the noble gases. For every thousand-million atoms of air, thereare only 87 atoms of xenon. Even so, it is recovered in commercial amounts by boiling off thexenon from fractional distillation of liquid air. Small amounts of xenon have been found insome minerals and meteorites, but not in amounts great enough to exploit.
Characteristics
Xenon is noncombustible, and even though it is considered inert, it will combine with afew elements (i.e., oxygen, fluorine, and platinum). Xenon is the only member of group 18that exhibits all of the even valence states of +2, +4, +6, and +8. It has similar oxidation stateseven though most periodic tables list a single oxidation state of zero.
Definition
A colorless odorless
monatomic element of the rare-gas group.
It occurs in trace amounts in air. Xenon is
used in electron tubes and strobe lighting.
Symbol: Xe; m.p. –111.9°C; b.p.
–107.1°C; d. 5.8971 (0°C) kg m–3; p.n. 54;
r.a.m. 131.29.
Definition
xenon: Symbol Xe. A colourless odourless gas belonging to group 18 of the periodic table (see noble gases); a.n. 54; r.a.m. 131.30; d. 5.887 g dm–3; m.p. –111.9°C; b.p. –107.1°C. It is present in the atmosphere (0.00087%) from which it is extracted by distillation of liquid air. There are nine natural isotopes with mass numbers 124, 126, 128–132, 134, and 136. Seven radioactive isotopes are also known. The element is used in Ûuorescent lamps and bubble chambers. Liquid xenon in a supercritical state at high temperatures is used as a solvent for infrared spectroscopy and for chemical reactions. The compound Xe+PtF6– was the Ürst noblegas compound to be synthesized. Several other compounds of xenon are known, including XeF2, XeF4, XeSiF6, XeO2F2, and XeO3. Recently, compounds have been isolated that contain xenon–carbon bonds, such as [C6H5Xe][B(C6H5)3F] (pentafluorophenylxenon fluoroborate), which is stable under normal conditions. The element was discovered in 1898 by Ramsey and Travers.
General Description
Xenon is an inert gas that is nonflammable and nonexplosive.The outer shell of xenon is complete thus it is not ahighly reactive compound neither seeking, nor donatingelectrons to biological molecules. Despite its “inert” status,xenon has been shown to interact with biological moleculesby forming an induced dipole in the presence of a cationicsite. An induced dipole could also result from an interactionwith another fleeting dipole formed at the proposedbinding site to form an induced dipole-induced dipole orLondon dispersion force.The mechanism of xenon anesthesiaand the site of action are still unknown.
Hazard
As a noble gas that is mostly inert, xenon is nontoxic and noncombustible. Some of itscompounds are toxic and potentially explosive, but there is little chance of coming into contactwith them on a day-to-day basis.
Industrial uses
Xenon, another gas occurring in the air to theextent of 1 part in 11 million, is the heaviestof the rare gases. When atomic reactors are operated at high power, xenon tends to buildup as a reaction product, poisoning the fuel andreducing the reactivity. Xenon lamps for militaryuse give a clear white light known as sunlightplus north-sky light. This color does notchange with the voltage, and thus the lampsrequire no voltage regulators. Xenon is a mildanesthetic; the accumulation from air helps toinduce natural sleep, but it cannot be used insurgery since the quantity needed producesasphyxiation.
Materials Uses
Gaseous neon, krypton, and xenon are noncorrosive and inert, so they may be contained in
systems constructed of any common metals de�signed to withstand safely the pressures involved. At the temperatures encountered with
liquid neon, krypton, and xenon, ordinary car�bon steels and most alloy steels lose their ductility and are considered unsafe for use with
these cryogenic liquids. Satisfactory materials
for use with liquid neon, krypton, and xenon
include austenitic stainless steel (for example
types 304 and 316) and other nickel-chromium
alloys, copper, Monel, brass, and aluminum.
Physiological effects
Neon, krypton, and xenon are nontoxic and
largely inert. They can act as simple asphyxiants
by displacing air, thereby diluting the concen�tration of oxygen below levels necessary to sup�port life. Inhalation in excessive concentrations
can result in dizziness, nausea, vomiting, loss of
consciousness, and death. Death may result
from errors in judgment, confusion, or loss of
consciousness, which prevents self-rescue. At
low-oxygen concentrations, unconsciousness
and death may occur in seconds without warn�ing.
storage
Gaseous neon, krypton, and xenon must be han�dled with all the precautions necessary for
safety with any nonflammable, nontoxic com�pressed gas.
All precautions necessary for the safe han�dling of any gas liquefied at very low tempera�tures must be observed with liquid neon, kryp�ton, and xenon. Extensive tissue damage or
bums can result from exposure to liquid neon,
krypton, or xenon or their cold vapors.
CGA P-l, Safe Handling of Compressed
Gases in Containers, provides basic guidelines
and requirements for the safe handling and stor�age of compressed gas cylinders. Also
refer to CGA P-12, Safe Handling of Cryogenic
Liquids, for information concerning safe han�dling of neon, krypton, and xenon in liquid form. Another useful reference concerning inert
gases is CGA P-14, Accident Prevention in
Oxygen-Rich and Oxygen-Deficient Atmos�pheres.
Waste Disposal
When disposal becomes necessary, vent neon,
krypton, and xenon gas slowly to a
well-ventilated outdoor location remote from
personnel work areas and building air intakes.
Do not dispose of any residual neon, krypton,
and xenon in compressed gas cylinders. Return
cylinders to the supplier with residual pressure,
the cylinder valve tightly closed, and the valve
caps in place.
Allow liquid neon, krypton, and xenon to
evaporate in well-ventilated outdoor locations
that are remote from work areas.
GRADES AVAILABLE
Neon, krypton, and xenon are available in vari�ous grades for industrial, medical, and advanced
technology uses. Neon is available with a mini�mum purity ranging from 75 mole percent to
99.999 mole percent. Krypton and xenon are
each available with a minimum purity ranging
from 99.95 mole percent to 99.997 mole per�cent.