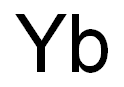| Definition |
A metallic element. A rare-earth metal of yttrium
subgroup, atomic number 70, aw 173.04, valence of
2, 3; exists in α and β forms, the latter being semiconductive
at pressures above 16,000 atm. There
are seven natural isotopes. |
| History |
Marignac in 1878 discovered a new component,
which he called ytterbia, in the Earth then known as erbia. In
1907, Urbain separated ytterbia into two components, which
he called neoytterbia and lutecia. The elements in these earths
are now known as ytterbium and lutetium, respectively. These
elements are identical with aldebaranium and cassiopeium,
discovered independently and at about the same time by von
Welsbach. Ytterbium occurs along with other rare earths in a
number of rare minerals. It is commercially recovered principally
from monazite sand, which contains about 0.03%. Ion-exchange
and solvent extraction techniques developed in recent
years have greatly simplified the separation of the rare earths
from one another. The element was first prepared by Klemm
and Bonner in 1937 by reducing ytterbium trichloride with
potassium. Their metal was mixed, however, with KCl. Daane,
Dennison, and Spedding prepared a much purer form in 1953
from which the chemical and physical properties of the element
could be determined. Ytterbium has a bright silvery luster, is
soft, malleable, and quite ductile. While the element is fairly
stable, it should be kept in closed containers to protect it from
air and moisture. Ytterbium is readily attacked and dissolved by
dilute and concentrated mineral acids and reacts slowly with
water. Ytterbium has three allotropic forms with transformation
points at –13° and 795°C. The beta form is a room-temperature,
face-centered, cubic modification, while the high-temperature
gamma form is a body-centered cubic form. Another bodycentered
cubic phase has recently been found to be stable at
high pressures at room temperatures. The beta form ordinarily
has metallic-type conductivity, but becomes a semiconductor
when the pressure is increased above 16,000 atm. The electrical resistance increases tenfold as the pressure is increased to
39,000 atm and drops to about 80% of its standard temperature-
pressure resistivity at a pressure of 40,000 atm. Natural ytterbium
is a mixture of seven stable isotopes. Twenty-six other
unstable isotopes and isomers are known. Ytterbium metal has
possible use in improving the grain refinement, strength, and
other mechanical properties of stainless steel. One isotope is
reported to have been used as a radiation source as a substitute
for a portable X-ray machine where electricity is unavailable.
Few other uses have been found. Ytterbium metal is available
with a purity of about 99.9% for about $10/g. Ytterbium has a
low acute toxicity rating. |