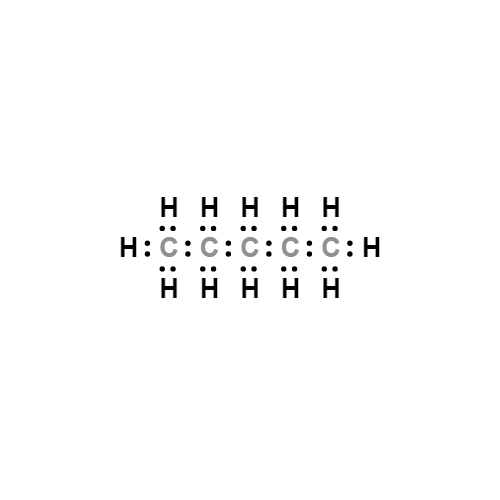
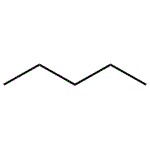
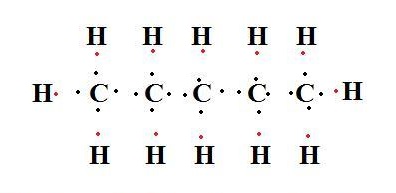Pentane
- Product NamePentane
- CAS109-66-0
- CBNumberCB6288989
-
MFC5H12
Lewis structure
- MW72.15
- EINECS203-692-4
- MDL NumberMFCD00009498
- MOL File109-66-0.mol
- MSDS FileSDS
Chemical Properties
| Melting point | -130 °C |
| Boiling point | 36 °C |
| Density | 0.626 g/mL at 25 °C(lit.) |
| vapor density | 2.48 (vs air) |
| vapor pressure | 26.98 psi ( 55 °C) |
| refractive index | n |
| Flash point | −57 °F |
| storage temp. | Store at +5°C to +30°C. |
| solubility | ethanol: soluble(lit.) |
| form | Liquid |
| pka | >14 (Schwarzenbach et al., 1993) |
| color | Colorless |
| Specific Gravity | 0.63 |
| Relative polarity | 0.009 |
| Odor | Like a gasoline. |
| Odor Threshold | 1.4ppm |
| explosive limit | 1.4-8%(V) |
| Evaporation Rate | 28.6 |
Safety
| Symbol(GHS) |
   
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H225-H304-H336-H411 | |||||||||
| Precautionary statements | P210-P273-P301+P310+P331 | |||||||||
| Hazard Codes | F+,Xn,N,F | |||||||||
| Risk Statements | 12-51/53-65-66-67 | |||||||||
| Safety Statements | 9-16-29-33-61-62 | |||||||||
| RIDADR | UN 1265 3/PG 2 | |||||||||
| WGK Germany | 2 | |||||||||
| RTECS | RZ9450000 | |||||||||
| F | 3-10 | |||||||||
| Autoignition Temperature | 500 °F | |||||||||
| TSCA | Yes | |||||||||
| HazardClass | 3 | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29011090 | |||||||||
| Hazardous Substances Data | 109-66-0(Hazardous Substances Data) | |||||||||
| Toxicity | LC (in air) in mice: 377 mg/l (Fühner) | |||||||||
| IDLA | 1,500 ppm [10% LEL] | |||||||||
| NFPA 704: |
|