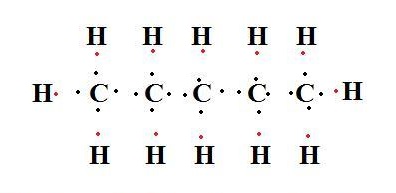Pentane is a colorless liquid. Gas above 36�C.
Gasoline-like odor.
n-Pentane is a flammable liquid. It has applications in industry as an aerosol propellant and as an important component of engine fuel.
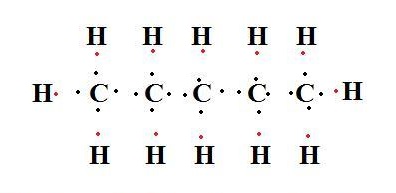
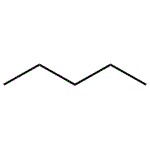
pentane lewis structure
N-propane is a CNS depressant. Studies with dogs have indicated that it induces cardiac sensitization. In high concentrations it causes incoordination and inhibition of the righting refl exes.
N-pentane is being used primarily in Europe in integral-skin flexible foams. Pentane is twice as effective as CFC-11 as a blowing agent.
Clear, colorless, volatile liquid with an odor resembling gasoline. An odor threshold concentration
of 1.4 ppmv was reported by Nagata and Takeuchi (1990).
Fuel; solvent; chemical synthesis
n-Pentane is a flammable liquid. It has diverse uses in industry—for instance, as an aerosol propellant and as an important component of engine fuel. n-Pentane is a CNS depressant. Laboratory studies in dogs indicate that prolonged exposure to high concentrations of n-pentane induces cardiac sensitization, poor coordination, and inhibition of the righting reflexes. NIOSH has recommended limits of n-pentane for working areas.
n-Pentane occurs in volatile petroleum fractions(gasoline) and as a constituent ofpetroleum ether. It is used as a solvent, in themanufacture of low-temperature thermometers,and as a blowing agent for plastics.
pentane: A straight-chain alkanehydrocarbon, C5H12; r.d. 0.63; m.p.–129.7°C; b.p. 36.1°C. It is obtainedby distillation of petroleum.
Pentane is produced by fractional distillation of natural gas
liquids and crude oil. It is also produced by the catalytic
crackdown of naphtha.
A straightchain
alkane obtained by distillation of
crude oil.
A clear colorless liquid with a petroleum-like odor. Flash point 57°F. Boiling point 97°F. Less dense than water and insoluble in water. Hence floats on water. Vapors are heavier than air.
Highly flammable. Insoluble in water.
Pentane is incompatible with strong oxidizers. Pentane is also incompatible with strong acids, alkalis, and oxygen. Mixtures with chlorine gas have produced explosions. Pentane will attack some forms of plastics, rubber, and coatings. .
n-Pentane did not exhibit any marked toxicityin animals. However, inhalation ofits vapors at high concentrations can causenarcosis and irritation of the respiratorypassages. Such effects may be observedwithin the range 5–10% concentration inair. In humans, inhalation of 5000 ppm for10 minutes did not cause respiratory tractirritation or other symptoms (Patty and Yant1929).
There is no report in the literature indicatingany adverse effects from pentaneother than narcosis and irritation. An intravenousLD50 value in mouse is recorded as446 mg/kg (NIOSH 1986).
Behavior in Fire: Containers may explode
Flammability and Explosibility
Extremely flammable
Reactivity with Water No reaction; Reactivity with Common Materials: No reaction; Stability During Transport: Stable; Neutralizing Agents for Acids and Caustics: Not pertinent; Polymerization: Not pertinent; Inhibitor of Polymerization: Not pertinent.
Moderately toxic by inhalation and intravenous routes. Narcotic in high concentration. The liquid can cause blisters on contact. Flammable liquid. Highly dangerous fire hazard when exposed to heat, flame, or oxidizers. Severe explosion hazard when exposed to heat or flame. Shock can shatter metal containers and release contents. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes
Pentane is used in manufacture of ice,
low-temperature thermometers; in solvent extraction
processes; as a blowing agent in plastics; as a fuel; as a
chemical intermediate (for amylchlorides, e.g.).
Schauer et al. (1999) reported pentane in a diesel-powered medium-duty truck exhaust at
an emission rate of 1,860 μg/km.
A constituent in gasoline. Harley et al. (2000) analyzed the headspace vapors of three grades of
unleaded gasoline where ethanol was added to replace methyl tert-butyl ether. The gasoline vapor
concentrations of pentane in the headspace were 14.2 wt % for regular grade, 12.6 wt % for midgrade,
and 9.3 wt % for premium grade.
California Phase II reformulated gasoline contained pentane at a concentration of 27.6 g/kg.
Gas-phase tailpipe emission rates from gasoline-powered automobiles with and without catalytic
converters were 4.29 and 536 mg/km, respectively (Schauer et al., 2002).
Schauer et al. (2001) measured organic compound emission rates for volatile organic compounds, gas-phase semi-volatile organic compounds, and particle-phase organic compounds
from the residential (fireplace) combustion of pine, oak, and eucalyptus. The gas-phase emission
rate of pentane was 4.7 mg/kg of pine burned. Emission rates of pentane were not measured during
the combustion of oak and eucalyptus.
Biological. n-Pentane may biodegrade in two ways. The first is the formation of pentyl
hydroperoxide, which decomposes to 1-pentanol followed by oxidation to pentanoic acid. The
other pathway involves dehydrogenation to 1-pentene, which may react with water giving 1-
pentanol (Dugan, 1972). Microorganisms can oxidize alkanes under aerobic conditions (Singer
and Finnerty, 1984). The most common degradative pathway involves the oxidation of the
terminal methyl group forming 1-pentanol. The alcohol may undergo a series of dehydrogenation
steps forming an aldehyde (valeraldehyde) then a fatty acid (valeric acid). The fatty acid may then
be metabolized by β-oxidation to form the mineralization products, carbon dioxide and water
(Singer and Finnerty, 1984). Mycobacterium smegnatis was capable of degrading pentane to 2-
pentanone (Riser-Roberts, 1992).
Photolytic. When synthetic air containing gaseous nitrous acid and pentane was exposed to
artificial sunlight (λ = 300–450 nm) methyl nitrate, pentyl nitrate, peroxyacetal nitrate, and
peroxypropionyl nitrate formed as products (Cox et al., 1980).
Chemical/Physical. Complete combustion in air yields carbon dioxide and water. Pentane will
not hydrolyze because it does not contain a hydrolyzable functional group.
UN1265 Pentanes Hazard Class: 3; Labels:
3-Flammable liquid.
Stir the pentane with successive portions of conc H2SO4 until there is no further coloration during 12hours, then with 0.5N KMnO4 in 3M H2SO4 for 12hours, wash with water and aqueous NaHCO3. Dry it with MgSO4 or Na2SO4, then P2O5 and fractionally distil it through a column packed with glass helices. It is also purified by passage through a column of silica gel, followed by distillation and storage with sodium hydride. An alternative purification is by azeotropic distillation with MeOH, which is subsequently washed out from the distillate (using water), followed by drying and re-distilling. For removal of carbonyl-containing impurities, see n-heptane. Also purify it by fractional freezing (ca 40%) on a copper coil through which cold air is passed, then wash with conc H2SO4 and fractionally distil it. [Beilstein 1 IV 303.]
The mechanism of toxicity is suspected to be similar to other
solvents that rapidly induce anesthesia-like effects, that is
a ‘nonspecific narcosis’ due to disruption (solvation) of the
integrity of the cellular membranes of the central nervous
system. The effect is similar to the ‘high’ experienced upon
exposure to other aliphatic hydrocarbon solvents.
As seen with other short-chain alkanes, upon inhalation,
pentane is moderately toxic and may cause irritation of the
respiratory tract and narcosis. The narcotic action of pentane
(observed following 1-h exposure to 90 000–120 000 ppm) is,
however, much less pronounced than effects seen following
exposure to the C1–C4 alkanes. Although the actual biochemical
mechanismof toxicity has not been discerned, the narcotic effects
seen are most likely related to its physical solvent properties.
Vapors may form explosive mixture with
air. Incompatible with oxidizers (chlorates, nitrates, peroxides,
permanganates, perchlorates, chlorine, bromine, fluorine,
etc.); contact may cause fires or explosions. Keep
away from alkaline materials, strong bases, strong acids,
oxoacids, epoxides. Attacks some plastics, rubbers, and
coatings.
Dissolve or mix the
material with a combustible solvent and burn in a chemical
incinerator equipped with an afterburner and scrubber.
All federal, state, and local environmental regulations
must be observed.