Amides
Amides refer to compounds derived from the substitution of the hydrogen group on the nitrogen atom of ammonia or an amine with an acyl group. Amides can also be seen as the compound derived from substitution of the hydroxyl group of the carboxylic acid molecules with amino or amino phenyl group. Common includes formamide (HCO-NH2), acetamide [CH3-CO-NH2] and carbonamide [CO- (NH2) 2]. At room temperature, they are all crystal except formamide which is in the form of crystal. Low amide is easily soluble in water. Amides are neutral substances with heating boiling of amide and water resulting in the occurrence of the hydrolysis reaction, producing carboxylic acid and ammonia, RCONH2 + H2O-- (Heating) - RCOOH + NH3. If we supplement alkali upon the hydrolysis, the generated acid will become a salt and the ammonia will escape. Upon heating: RCONH2 + NaOH - (heating) - RCOONa + NH3↑.
Polypeptides, proteins, and synthetic fibers such as nylon 6, nylon 66 and nylon 1010 are all compounds containing amide bonds. The structural formula is the common amide in vivo including glutamine (abbreviated as GLn or Q) and asparagine (abbreviated as Asn or N) and their derivatives. They not only are the composition of protein, but also play the role of nitrogen storage and nitrogen supply in the life activities. For example, part of the nitrogen during the synthesis of purine and pyrimidine nucleotides can be supplied by glutamine. It is often also the bridge linking the protein and carbohydrates in the glycoproteins.
The products derived from substitution of the two hydrogen atoms in the ammonia molecules by acyl groups are called imide. The hydrogen atom attached to the nitrogen atom in the imide molecule can be removed in the form of a proton, and the imide is weakly acidic.
Many Amides have industrial applications. For example, acetamide (CH3CONH2), dimethylformamide [HCON (CH3) 2] is an important solvent. Acetanilide is the intermediates for the preparation of sulfa drugs. Urea is an important raw material for nitrogen and synthetic resins. Amide type with ring structure is called lactam. The important lactam is caprolactam, which is the monomer for the synthesis of nylon 6. Application of cyclohexanone as raw material can be used for preparation of caprolactam.
- Structure:
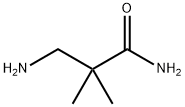
- Chemical Name:3-Amino-2,2-dimethylpropionamide
- CAS:324763-51-1
- MF:C5H12N2O
- Structure:
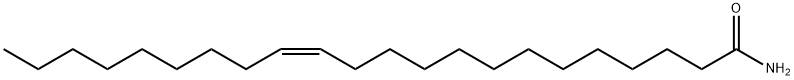
- Chemical Name:Erucamide
- CAS:112-84-5
- MF:C22H43NO
- Structure:
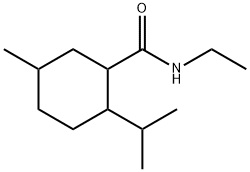
- Chemical Name:N-Ethyl-p-menthane-3-carboxamide
- CAS:39711-79-0
- MF:C13H25NO
- Structure:
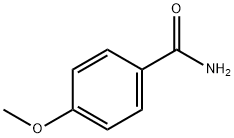
- Chemical Name:4-Methoxybenzamide
- CAS:3424-93-9
- MF:C8H9NO2
- Structure:
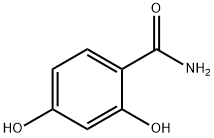
- Chemical Name:2,4-Dihydroxybenzamide
- CAS:3147-45-3
- MF:C7H7NO3
- Structure:
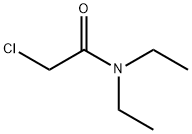
- Chemical Name:2-Chloro-N,N-diethylacetamide
- CAS:2315-36-8
- MF:C6H12ClNO
- Structure:
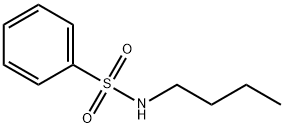
- Chemical Name:N-n-Butyl benzene sulfonamide
- CAS:3622-84-2
- MF:C10H15NO2S
- Structure:

- Chemical Name:N,N'-Ethylenebis(stearamide)
- CAS:110-30-5
- MF:C38H76N2O2
- Structure:
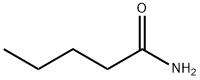
- Chemical Name:Pentanamide
- CAS:626-97-1
- MF:C5H11NO
- Structure:
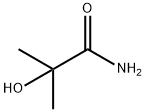
- Chemical Name:2-Methyl-2-hydroxypropionamide
- CAS:13027-88-8
- MF:C4H9NO2
- Structure:
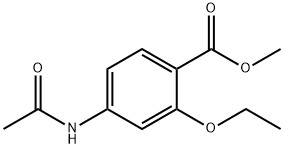
- Chemical Name:Ethopabate
- CAS:59-06-3
- MF:C12H15NO4
- Structure:
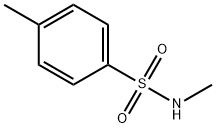
- Chemical Name:N-Methyl-p-toluenesulfonamide
- CAS:640-61-9
- MF:C8H11NO2S
- Structure:
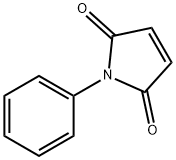
- Chemical Name:N-Phenylmaleimide
- CAS:941-69-5
- MF:C10H7NO2
- Structure:
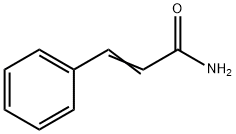
- Chemical Name:Cinnamamide
- CAS:621-79-4
- MF:C9H9NO
- Structure:
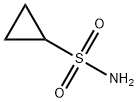
- Chemical Name:Cyclopropanesulfonamide
- CAS:154350-29-5
- MF:C3H7NO2S
- Structure:
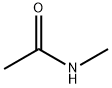
- Chemical Name:N-Methylacetamide
- CAS:79-16-3
- MF:C3H7NO
- Structure:
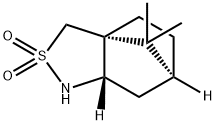
- Chemical Name:(2S)-Bornane-10,2-sultam
- CAS:108448-77-7
- MF:C10H17NO2S
- Structure:
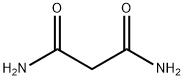
- Chemical Name:Malonamide
- CAS:108-13-4
- MF:C3H6N2O2
- Structure:
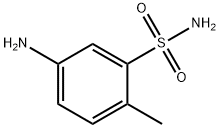
- Chemical Name:5-Amino-2-methylbenzenesulfonamide
- CAS:6973-09-7
- MF:C7H10N2O2S
- Structure:
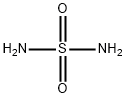
- Chemical Name:Sulfamide
- CAS:7803-58-9
- MF:H4N2O2S
- Structure:

- Chemical Name:N,N-Dimethylformamide
- CAS:68-12-2
- MF:C3H7NO
- Structure:
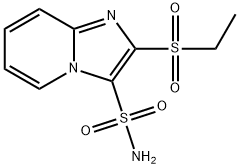
- Chemical Name:2-Ethylsulfonylimidazo[1,2-a]pyridine-3-sulfonamide
- CAS:141776-47-8
- MF:C9H11N3O4S2
- Structure:
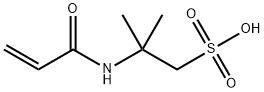
- Chemical Name:2-Acrylamide-2-methylpropanesulfonic acid
- CAS:15214-89-8
- MF:C7H13NO4S
- Structure:
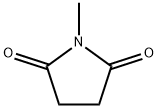
- Chemical Name:N-METHYLSUCCINIMIDE
- CAS:1121-07-9
- MF:C5H7NO2
- Structure:
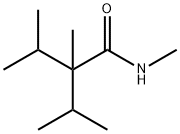
- Chemical Name:2-Isopropyl-N,2,3-trimethylbutyramide
- CAS:51115-67-4
- MF:C10H21NO
- Structure:
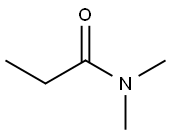
- Chemical Name:N,N-Dimethylpropionamide
- CAS:758-96-3
- MF:C5H11NO
- Structure:
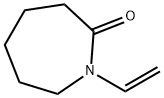
- Chemical Name:N-Vinylcaprolactam
- CAS:2235-00-9
- MF:C8H13NO
- Structure:
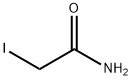
- Chemical Name:2-Iodoacetamide
- CAS:144-48-9
- MF:C2H4INO
- Structure:
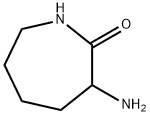
- Chemical Name:DL-ALPHA-AMINO-EPSILON-CAPROLACTAM
- CAS:671-42-1
- MF:C6H12N2O
- Structure:
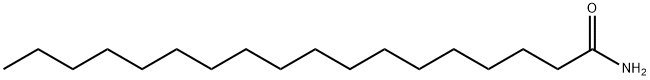
- Chemical Name:Octadecanamide
- CAS:124-26-5
- MF:C18H37NO
- Structure:
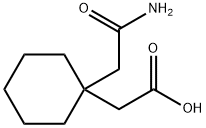
- Chemical Name:1,1-Cyclohexanediacetic acid mono amide
- CAS:99189-60-3
- MF:C10H17NO3
- Structure:
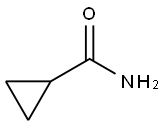
- Chemical Name:CYCLOPROPANECARBOXAMIDE
- CAS:6228-73-5
- MF:C4H7NO
- Structure:
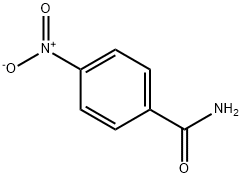
- Chemical Name:p-Nitrobenzamide
- CAS:619-80-7
- MF:C7H6N2O3
- Structure:
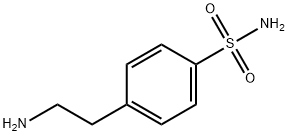
- Chemical Name:4-(2-Aminoethyl)benzenesulfonamide
- CAS:35303-76-5
- MF:C8H12N2O2S
- Structure:
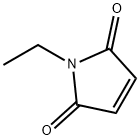
- Chemical Name:N-Ethylmaleimide
- CAS:128-53-0
- MF:C6H7NO2
- Structure:
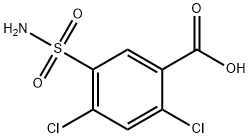
- Chemical Name:2,4-Dichloro-5-sulfamoylbenzoic acid
- CAS:2736-23-4
- MF:C7H5Cl2NO4S
- Structure:
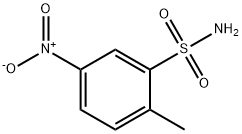
- Chemical Name:2-Methyl-5-nitrobenzenesulfonamide
- CAS:6269-91-6
- MF:C7H8N2O4S
- Structure:
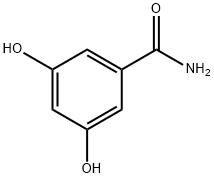
- Chemical Name:3,5-Dihydroxybenzamide
- CAS:3147-62-4
- MF:C7H7NO3
- Structure:
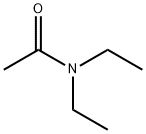
- Chemical Name:Diethylacetamide
- CAS:685-91-6
- MF:C6H13NO
- Structure:
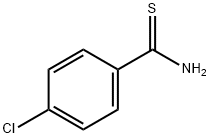
- Chemical Name:4-Chlorothiobenzamide
- CAS:2521-24-6
- MF:C7H6ClNS
- Structure:
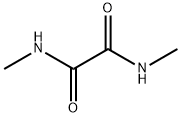
- Chemical Name:N,N'-Dimethyloxalamide
- CAS:615-35-0
- MF:C4H8N2O2
- Structure:
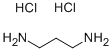
- Chemical Name:1,3-DIAMINOPROPANE DIHYDROCHLORIDE
- CAS:10517-44-9
- MF:C3H12Cl2N2
- Structure:
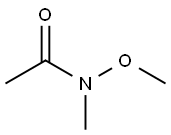
- Chemical Name:N-Methoxy-N-methylacetamide
- CAS:78191-00-1
- MF:C4H9NO2
- Structure:
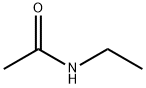
- Chemical Name:N-Ethylacetamide
- CAS:625-50-3
- MF:C4H9NO
- Structure:
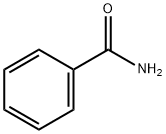
- Chemical Name:Benzamide
- CAS:55-21-0
- MF:C7H7NO
- Structure:
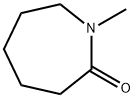
- Chemical Name:N-Methylcaprolactam
- CAS:2556-73-2
- MF:C7H13NO
- Structure:
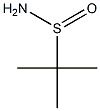
- Chemical Name:tert-Butanesulfinamide
- CAS:146374-27-8
- MF:C4H11NOS
- Structure:
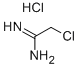
- Chemical Name:CHLOROACETAMIDINE HYDROCHLORIDE
- CAS:10300-69-3
- MF:C2H6Cl2N2
- Structure:
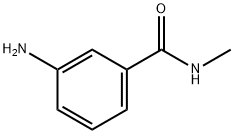
- Chemical Name:3-AMINOBENZOYLMETHYLAMIDE
- CAS:25900-61-2
- MF:C8H10N2O
- Structure:
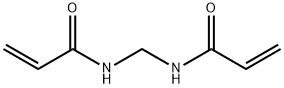
- Chemical Name:N,N'-Methylenebisacrylamide
- CAS:110-26-9
- MF:C7H10N2O2
- Structure:
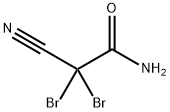
- Chemical Name:2,2-Dibromo-2-cyanoacetamide
- CAS:10222-01-2
- MF:C3H2Br2N2O
- Structure:
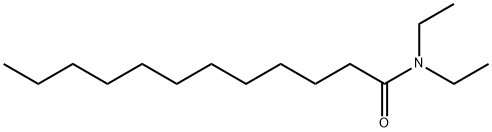
- Chemical Name:N,N-Diethyldodecanamide
- CAS:3352-87-2
- MF:C16H33NO
- Structure:
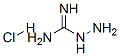
- Chemical Name:AMINOGUANIDINE HYDROCHLORIDE
- CAS:1937-19-5
- MF:CH7ClN4
- Structure:
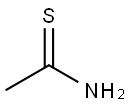
- Chemical Name:Thioacetamide
- CAS:62-55-5
- MF:C2H5NS
- Structure:
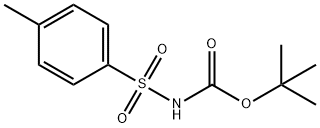
- Chemical Name:N-(TERT-BUTOXYCARBONYL)-P-TOLUENESULFONAMIDE
- CAS:18303-04-3
- MF:C12H17NO4S
- Structure:
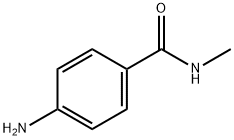
- Chemical Name:4-Amino-N-methylbenzamide
- CAS:6274-22-2
- MF:C8H10N2O
- Structure:
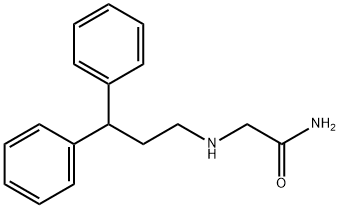
- Chemical Name:2-[(3,3-DIPHENYLPROPYL)AMINO]ACETAMIDE HYDROCHLORIDE
- CAS:76991-05-4
- MF:C17H20N2O
- Structure:
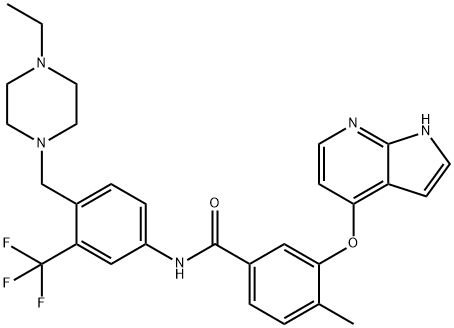
- Chemical Name:NG-25
- CAS:1315355-93-1
- MF:C29H30F3N5O2
- Structure:
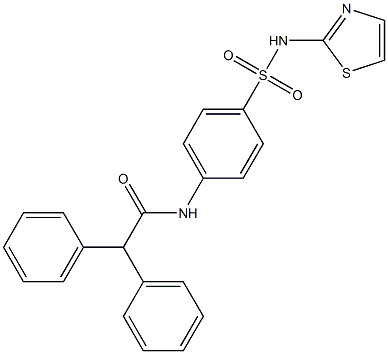
- Chemical Name:2,2-Diphenyl-N-[4-(thiazol-2-ylsulfamoyl)-phenyl]-acetamide
- CAS:313254-51-2
- MF:C23H19N3O3S2
- Structure:
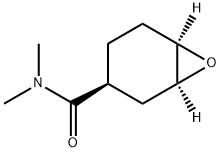
- Chemical Name:(1S,3S,6R)-N,N-dimethyl-7-oxabicyclo[4.1.0]heptane-3-carboxamide
- CAS:929693-35-6
- MF:C9H15NO2
- Structure:
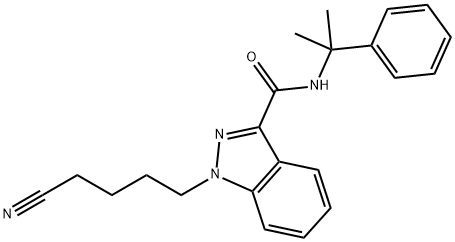
- Chemical Name:SGT-78
- CAS:1631074-54-8
- MF:C22H24N4O
- Structure:
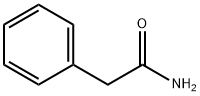
- Chemical Name:2-Phenylacetamide
- CAS:103-81-1
- MF:C8H9NO
- Structure:
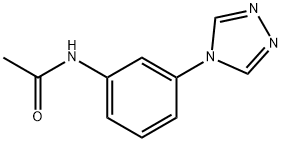
- Chemical Name:N-(3-(4H-1,2,4-triazol-4-yl)phenyl)acetamide
- CAS:1029769-35-4
- MF:C10H10N4O
- Structure:
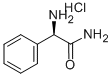
- Chemical Name:D-2-AMINO-2-PHENYLACETAMIDE
- CAS:63291-39-4
- MF:C8H10N2O.ClH
- Structure:
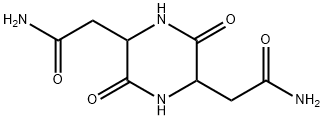
- Chemical Name:2,5-Piperazinediacetamide, 3,6-dioxo-
- CAS:98490-54-1
- MF:C8H12N4O4
- Structure:
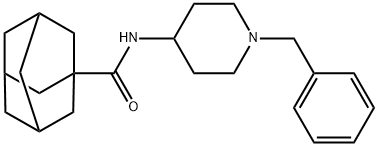
- Chemical Name:7]decane-1-carboxamide, N-[1-(phenylmethyl)-4-piperidinyl]
- CAS:314030-54-1
- MF:C23H32N2O
- Structure:
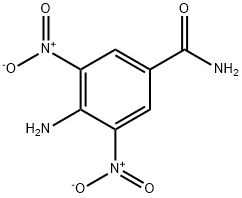
- Chemical Name:4-AMINO-3,5-DINITROBENZAMIDE
- CAS:54321-79-8
- MF:C7H6N4O5
- Structure:
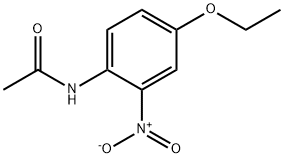
- Chemical Name:N-(4-ethoxy-2-nitrophenyl)acetamide
- CAS:885-81-4
- MF:C10H12N2O4
- Structure:
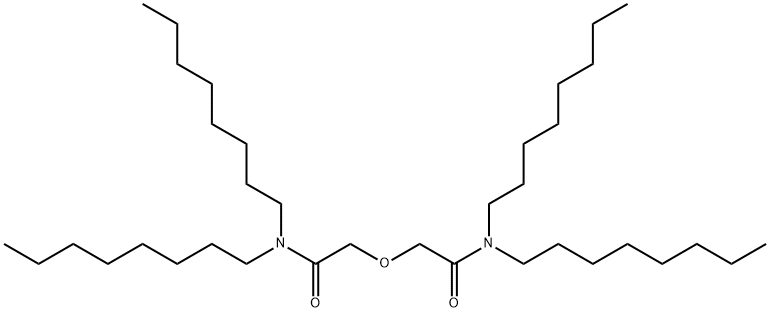
- Chemical Name:AcetaMide, 2,2'-oxybis[N,N-dioctyl-
- CAS:342794-43-8
- MF:C36H72N2O3
- Structure:
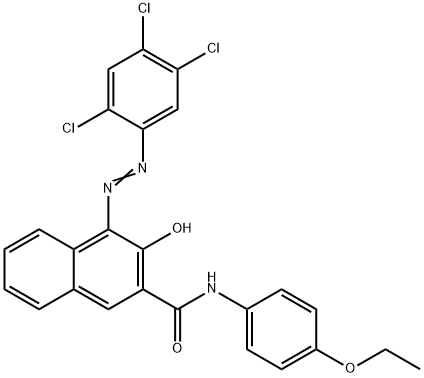
- Chemical Name:N-(4-ethoxyphenyl)-3-hydroxy-4-[(2,4,5-trichlorophenyl)azo]naphthalene-2-carboxamide
- CAS:5012-29-3
- MF:C25H18Cl3N3O3
- Structure:
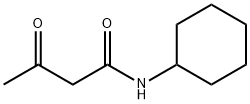
- Chemical Name:N-cyclohexylacetoacetamide
- CAS:1132-42-9
- MF:C10H17NO2
- Structure:
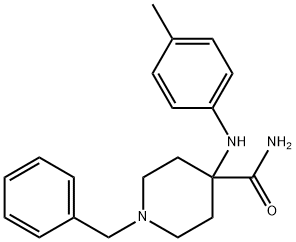
- Chemical Name:1-benzyl-4-(p-toluidino)piperidine-4-carboxamide
- CAS:1164-72-3
- MF:C20H25N3O
- Structure:
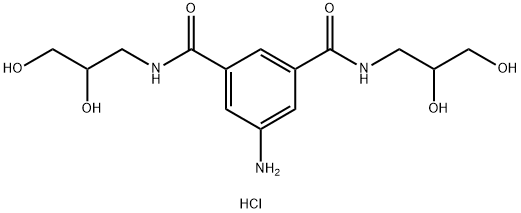
- Chemical Name:5-Amino-N,N'-bis(2,3-dihydroxypropyl)isophthalamide
- CAS:203515-86-0
- MF:C14H22ClN3O6
- Structure:
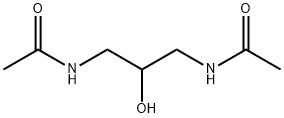
- Chemical Name:N,N'-(2-hydroxypropane-1,3-diyl)diacetamide
- CAS:261507-87-3
- MF:C7H14N2O3
- Structure:
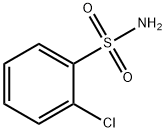
- Chemical Name:o-Chlorobenzenesulfonamide
- CAS:6961-82-6
- MF:C6H6ClNO2S
- Structure:
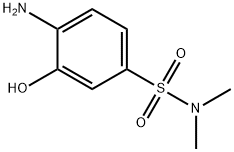
- Chemical Name:2-AMINOPHENOL-5-(N,N-DIMETHYL)SULFONAMIDE
- CAS:41608-75-7
- MF:C8H12N2O3S
- Structure:
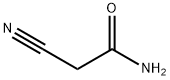
- Chemical Name:2-Cyanoacetamide
- CAS:107-91-5
- MF:C3H4N2O
- Structure:
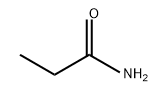
- Chemical Name:Propionamide
- CAS:79-05-0
- MF:C3H7NO
- Structure:
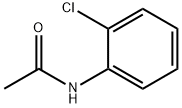
- Chemical Name:2'-Chloroacetanilide
- CAS:533-17-5
- MF:C8H8ClNO
- Structure:
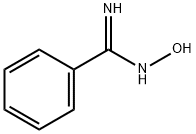
- Chemical Name:BENZAMIDOXIME HYDROCHLORIDE
- CAS:613-92-3
- MF:C7H8N2O
- Structure:
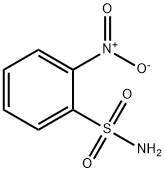
- Chemical Name:2-Nitrobenzenesulfonamide
- CAS:5455-59-4
- MF:C6H6N2O4S
- Structure:
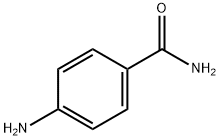
- Chemical Name:p-Aminobenzamide
- CAS:2835-68-9
- MF:C7H8N2O
- Structure:
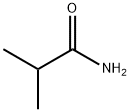
- Chemical Name:Isobutyramide
- CAS:563-83-7
- MF:C4H9NO
- Structure:
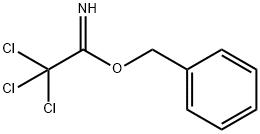
- Chemical Name:BENZYL 2,2,2-TRICHLOROACETIMIDATE
- CAS:81927-55-1
- MF:C9H8Cl3NO
- Structure:
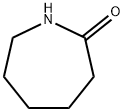
- Chemical Name:Caprolactam
- CAS:105-60-2
- MF:C6H11NO
- Structure:
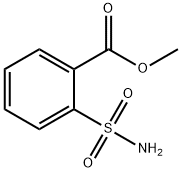
- Chemical Name:2-Carbomethoxybenzenesulfonamide
- CAS:57683-71-3
- MF:C8H9NO4S
- Structure:
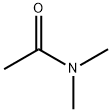
- Chemical Name:N,N-Dimethylacetamide
- CAS:127-19-5
- MF:C4H9NO
- Structure:
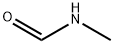
- Chemical Name:N-Methylformamide
- CAS:123-39-7
- MF:C2H5NO
- Structure:
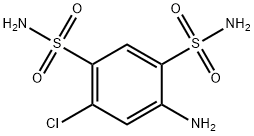
- Chemical Name:4-Amino-6-chlorobenzene-1,3-disulfonamide
- CAS:121-30-2
- MF:C6H8ClN3O4S2
- Structure:
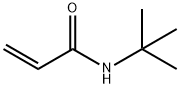
- Chemical Name:N-TERT-BUTYLACRYLAMIDE
- CAS:107-58-4
- MF:C7H13NO
- Structure:
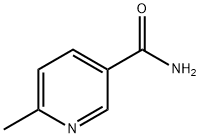
- Chemical Name:6-METHYLNICOTINAMIDE
- CAS:6960-22-1
- MF:C7H8N2O
- Structure:
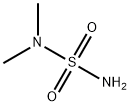
- Chemical Name:N,N-Dimethylsulfamide
- CAS:3984-14-3
- MF:C2H8N2O2S
- Structure:
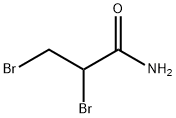
- Chemical Name:2,3-DIBROMOPROPIONAMIDE
- CAS:15102-42-8
- MF:C3H5Br2NO
- Structure:
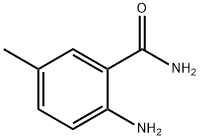
- Chemical Name:2-AMINO-5-METHYLBENZAMIDE
- CAS:40545-33-3
- MF:C8H10N2O
- Structure:
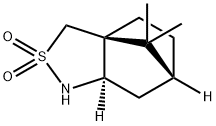
- Chemical Name:(2R)-Bornane-10,2-sultam
- CAS:94594-90-8
- MF:C10H17NO2S
- Structure:
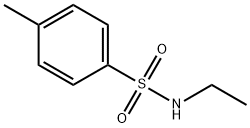
- Chemical Name:N-Ethyl-p-toluenesulfonamide
- CAS:80-39-7
- MF:C9H13NO2S
- Structure:
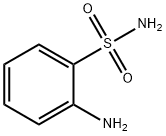
- Chemical Name:2-Aminobenzenesulfonamide
- CAS:3306-62-5
- MF:C6H8N2O2S
- Structure:

- Chemical Name:Formamide
- CAS:75-12-7
- MF:CH3NO
- Structure:
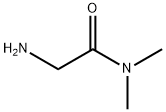
- Chemical Name:2-amino-N,N-dimethylacetamide
- CAS:1857-19-8
- MF:C4H10N2O