Crown ether
Crown ether, also known as "crown compound" because of its crown-like shape, is a class of compounds with a special property of complexation which was newly developed after 1970s. It’s a class of macrocyclic polyether composed of [CH2CH2Y] n (n> 2) units, wherein Y is a heteroatom such as O, N, S, or P. Crown ether narrowly refers to macrocyclic polyether in which Y is O, such as 18-crown-6 and dicyclohexyl-18-crown-6. They are named after the order of non-ring substituents, the number of the ring atoms, class name (crown) and the number of the ring hetero atoms according to the customary nomenclature, such as dibenzo-18-crown-6. Saturated crown ether is a colorless viscous liquid or low melt solid while aromatic ring-containing crown ether is a colorless crystal. Aromatic ring-containing crown ethers are insoluble in water, alcohol and common organic solvents at room temperature but more soluble in dichloromethane, chloroform, pyridine and formic acid. Crown ethers containing cyclohexyl have a higher solubility than the corresponding benzo crown ether in water, alcohols and aromatic hydrocarbons, also in petroleum ether.
Crown ether generally has a good thermal stability. However its ether bonds are susceptible to oxidation when melted or at a high temperature. The aromatic ring in the molecule can take part in bromination, nitration, esterification and ozonization, as well as form a polymer with formaldehyde in condensation. Crown ether can form a complex substance called crypate rendering many salts soluble in non-polar organic solvent, so that many of difficult reactions can be easily carried out. The stronger its lipophilic is, the greater catalytic activity it has. Furthermore, the reaction is selective since its fixed space can only accommodate ion of the corresponding size. The stability of formed by crown ethers and positive ions is mainly dependent on the relative size of the polyether ring and positive ions; and the stability constant is generally small for this crypate was formed by electrostatic interactions. What makes the reaction convenient is that the complexing can be easily broken down by adding water, dilute acid or through heat decomposition.
Preparation: Williamson (Williamson) ether synthesis is generally adopted. But in order to prevent a chain polymerization, high dilution method, fractional condensation and template reaction are recommended.
Currently the most widely used also the most readily available crown ethers are 18-crown-6, dibenzo-18-crown-6 and dicyclohexyl-18-crown-6. It is an important characteristic of crown ethers that it forms complexes with metal cations, which is utilized in phase transfer catalysis in organic synthesis.
- Structure:
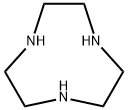
- Chemical Name:1,4,7-Triazacyclononane
- CAS:4730-54-5
- MF:C6H15N3
- Structure:
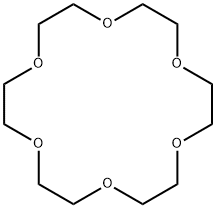
- Chemical Name:18-Crown-6
- CAS:17455-13-9
- MF:C12H24O6
- Structure:
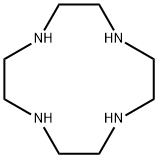
- Chemical Name:Cyclen
- CAS:294-90-6
- MF:C8H20N4
- Structure:

- Chemical Name:2,5-DIMETHOXYCINNAMIC ACID
- CAS:161282-95-7
- MF:C40H46O8
- Structure:
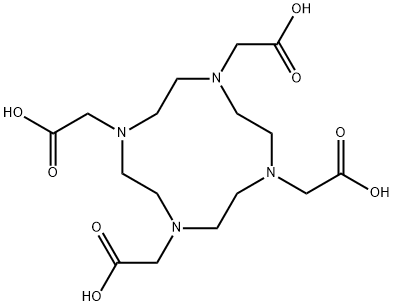
- Chemical Name:DOTA
- CAS:60239-18-1
- MF:C16H28N4O8
- Structure:
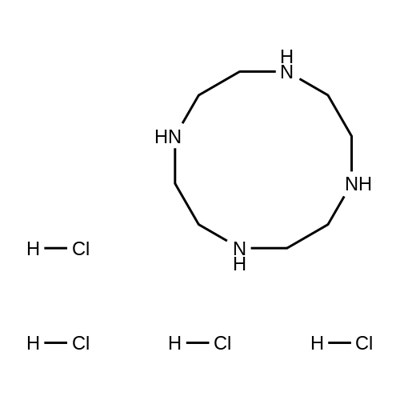
- Chemical Name:1,4,7,10-TETRAAZACYCLODODECANE TETRAHYDROCHLORIDE
- CAS:10045-25-7
- MF:C8H21ClN4
- Structure:
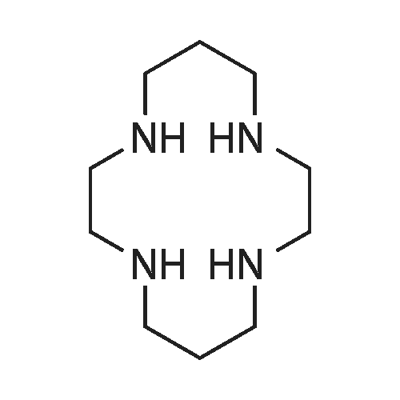
- Chemical Name:1,4,8,11-TETRAAZACYCLOTETRADECANE
- CAS:295-37-4
- MF:C10H24N4
- Structure:
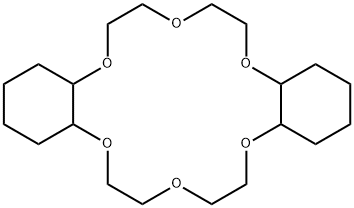
- Chemical Name:Dicyclohexano-18-crown-6
- CAS:16069-36-6
- MF:C20H36O6
- Structure:
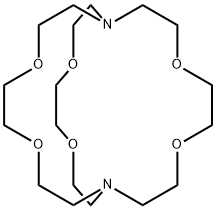
- Chemical Name:Kryptofix 222
- CAS:23978-09-8
- MF:C18H36N2O6
- Structure:
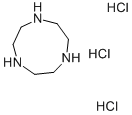
- Chemical Name:1,4,7-Triazacyclononane trihydrochloride
- CAS:58966-93-1
- MF:C6H18Cl3N3
- Structure:
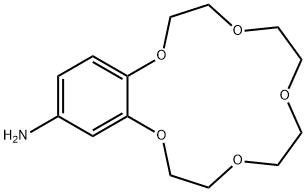
- Chemical Name:4'-Aminobenzo-15-crown-5-ether
- CAS:60835-71-4
- MF:C14H21NO5
- Structure:
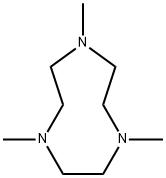
- Chemical Name:1,4,7-TRIMETHYL-1,4,7-TRIAZACYCLONONANE
- CAS:96556-05-7
- MF:C9H21N3
- Structure:
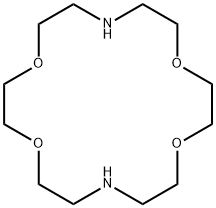
- Chemical Name:4,13-Diaza-18-crown 6-ether
- CAS:23978-55-4
- MF:C12H26N2O4
- Structure:
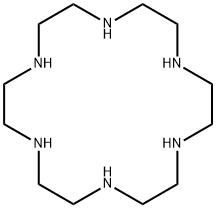
- Chemical Name:1,4,7,10,13,16-HEXAAZACYCLOOCTADECANE
- CAS:296-35-5
- MF:C12H30N6
- Structure:
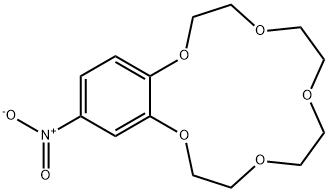
- Chemical Name:4-NITROBENZO-15-CROWN-5
- CAS:60835-69-0
- MF:C14H19NO7
- Structure:
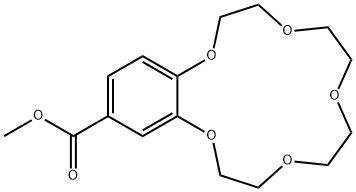
- Chemical Name:2,3-(4-METHOXYCARBONYLBENZO)-1,4,7,10,13-PENTAOXACYCLOPENTADEC-2-ENE
- CAS:56683-56-8
- MF:C16H22O7
- Structure:
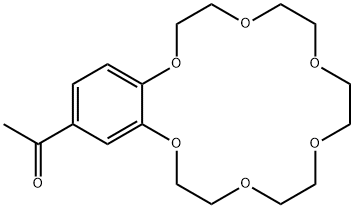
- Chemical Name:4'-ACETYLBENZO-18-CROWN 6-ETHER
- CAS:41855-35-0
- MF:C18H26O7
- Structure:
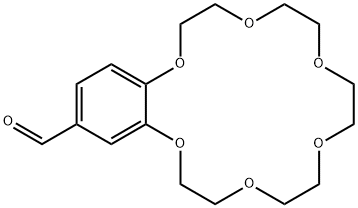
- Chemical Name:4'-FORMYLBENZO-18-CROWN 6-ETHER
- CAS:60835-74-7
- MF:C17H24O7
- Structure:
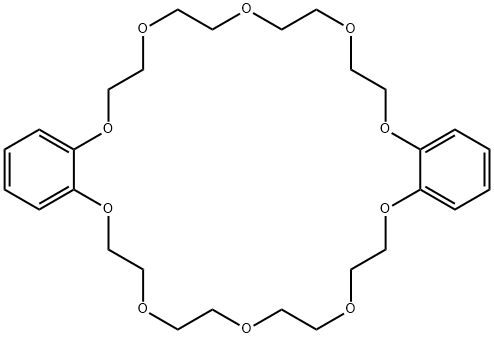
- Chemical Name:DIBENZO-30-CROWN-10
- CAS:17455-25-3
- MF:C28H40O10
- Structure:
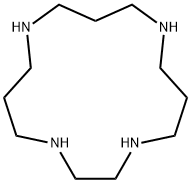
- Chemical Name:1,4,8,12-TETRAAZACYCLOPENTADECANE
- CAS:15439-16-4
- MF:C11H26N4
- Structure:
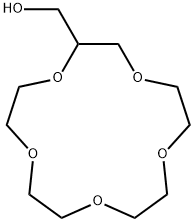
- Chemical Name:2-HYDROXYMETHYL-15-CROWN-5
- CAS:75507-25-4
- MF:C11H22O6
- Structure:
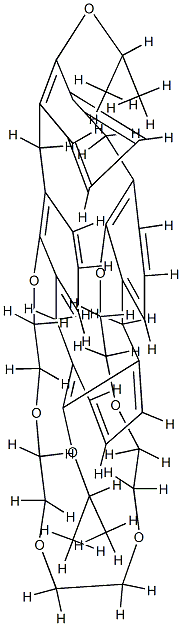
- Chemical Name:1,3-DIISOPROPOXYCALIX[4!ARENECROWN-6, 97
- CAS:161282-96-8
- MF:C44H54O8
- Structure:
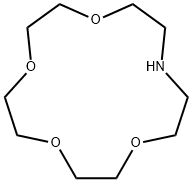
- Chemical Name:Aza-15-crown-5
- CAS:66943-05-3
- MF:C10H21NO4
- Structure:
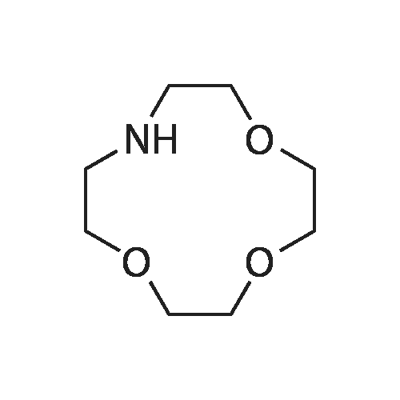
- Chemical Name:1,4,7-TRIOXA-10-AZACYCLODODECANE
- CAS:41775-76-2
- MF:C8H17NO3
- Structure:
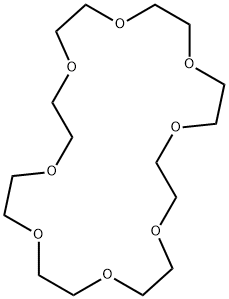
- Chemical Name:1,4,7,10,13,16,19,22-OCTAOXACYCLOTETRACOSANE
- CAS:33089-37-1
- MF:C16H32O8
- Structure:
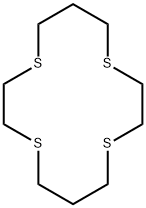
- Chemical Name:1,4,8,11-TETRATHIACYCLOTETRADECANE
- CAS:24194-61-4
- MF:C10H20S4
- Structure:
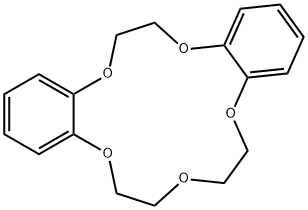
- Chemical Name:DIBENZO-15-CROWN-5
- CAS:14262-60-3
- MF:C18H20O5
- Structure:
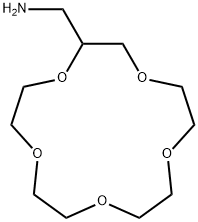
- Chemical Name:1,4,7,10,13-PENTAOXACYCLOPENTADECANE-2-METHANAMINE
- CAS:83585-56-2
- MF:C11H23NO5
- Structure:
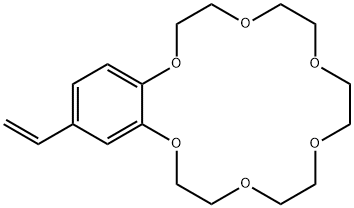
- Chemical Name:4-Vinylbenzo-18-crown-6
- CAS:39557-71-6
- MF:C18H26O6
- Structure:
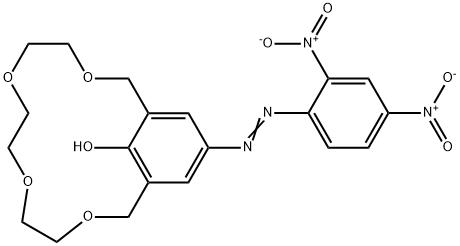
- Chemical Name:15-CROWN-4 [4-(2,4-DINITROPHENYLAZO)PHENOL]
- CAS:81238-57-5
- MF:C20H22N4O9
- Structure:
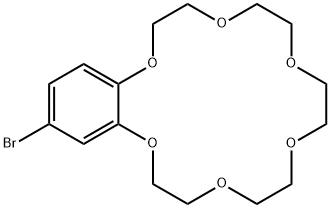
- Chemical Name:4'-BROMOBENZO-18-CROWN-6
- CAS:75460-28-5
- MF:C16H23BrO6
- Structure:
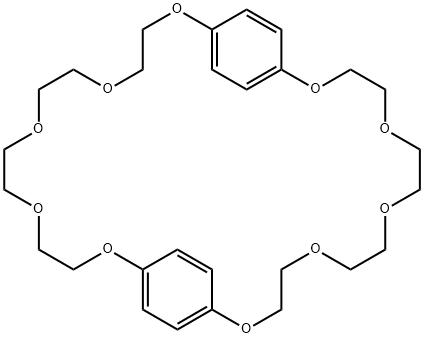
- Chemical Name:BIS(1,4-PHENYLENE)-34-CROWN 10-ETHER
- CAS:53914-95-7
- MF:C28H40O10
- Structure:
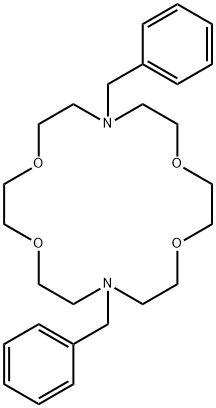
- Chemical Name:N,N'-DIBENZYL-1,4,10,13-TETRAOXA-7,16-DIAZACYCLOOCTADECANE
- CAS:69703-25-9
- MF:C26H38N2O4
- Structure:
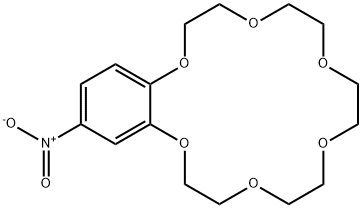
- Chemical Name:4-NITROBENZO-18-CROWN-6
- CAS:53408-96-1
- MF:C16H23NO8
- Structure:
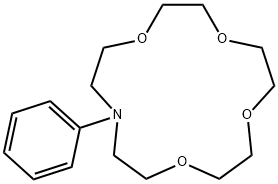
- Chemical Name:N-PHENYLAZA-15-CROWN 5-ETHER
- CAS:66750-10-5
- MF:C16H25NO4
- Structure:
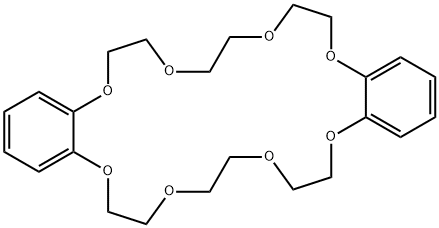
- Chemical Name:Dibenzo-24-crown-8
- CAS:14174-09-5
- MF:C24H32O8
- Structure:
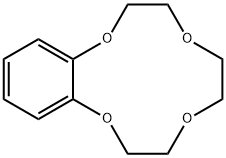
- Chemical Name:BENZO-12-CROWN-4
- CAS:14174-08-4
- MF:C12H16O4
- Structure:
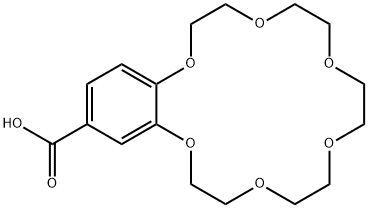
- Chemical Name:2,3-(4-CARBOXYBENZO)-1,4,7,10,13,16-HEXAOXACYCLOOCTADEC-2-ENE
- CAS:60835-75-8
- MF:C17H24O8
- Structure:
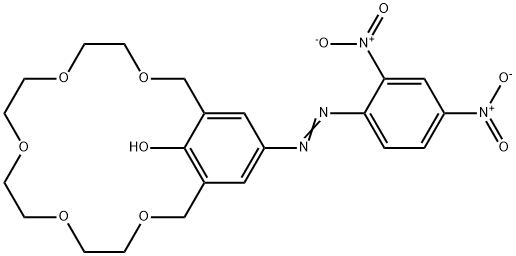
- Chemical Name:18-CROWN-5 [4-(2,4-DINITROPHENYLAZO)PHENOL]
- CAS:81238-58-6
- MF:C22H26N4O10
- Structure:
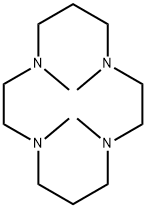
- Chemical Name:1,4,8,11-TETRAMETHYL-1,4,8,11-TETRAAZACYCLOTETRADECANE
- CAS:41203-22-9
- MF:C14H32N4
- Structure:
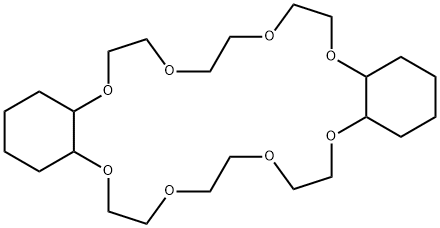
- Chemical Name:Dicyclohexano-24-crown-8
- CAS:17455-23-1
- MF:C24H44O8
- Structure:
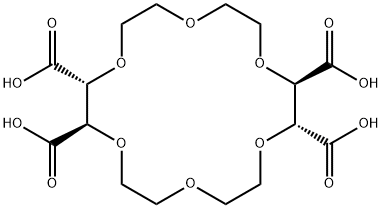
- Chemical Name:(+)-(18-CROWN-6)-2,3,11,12-TETRACARBOXYLIC ACID
- CAS:61696-54-6
- MF:C16H24O14
- Structure:
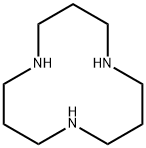
- Chemical Name:1,5,9-TRIAZACYCLODODECANE
- CAS:294-80-4
- MF:C9H21N3
- Structure:
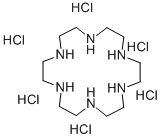
- Chemical Name:1,4,7,10,13,16-hexaazacyclooctadecane hexahydrochloride
- CAS:58105-91-2
- MF:C12H30N6.6HCl
- Structure:
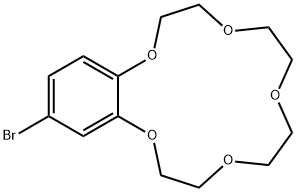
- Chemical Name:4'-BROMOBENZO-15-CROWN 5-ETHER
- CAS:60835-72-5
- MF:C14H19BrO5
- Structure:
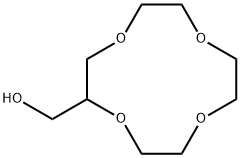
- Chemical Name:2-(HYDROXYMETHYL)-12-CROWN 4-ETHER
- CAS:75507-26-5
- MF:C9H18O5
- Structure:
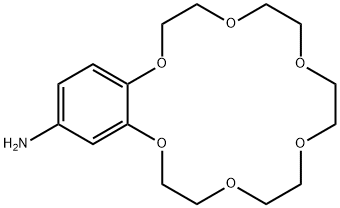
- Chemical Name:4'-Aminobenzo-18-crown-6
- CAS:68941-06-0
- MF:C16H25NO6
- Structure:
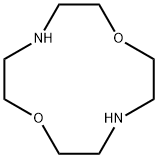
- Chemical Name:1,7-DIAZA-12-CROWN-4
- CAS:294-92-8
- MF:C8H18N2O2
- Structure:
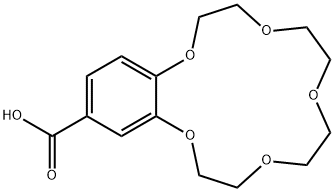
- Chemical Name:(BENZO-15-CROWN 5-ETHER)-4'-CARBOXYLIC ACID
- CAS:56683-55-7
- MF:C15H20O7
- Structure:
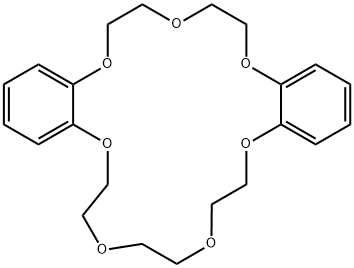
- Chemical Name:[2,5]-DIBENZO-21-CROWN-7
- CAS:14098-41-0
- MF:C22H28O7
- Structure:
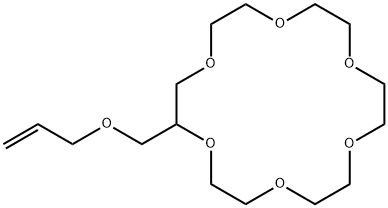
- Chemical Name:2-(ALLYLOXYMETHYL)-18-CROWN 6-ETHER
- CAS:84812-04-4
- MF:C16H30O7
- Structure:
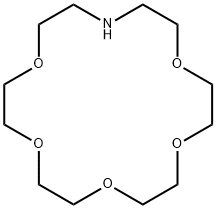
- Chemical Name:1,4,7,10,13-PENTAOXA-16-AZACYCLOOCTADECANE
- CAS:33941-15-0
- MF:C12H25NO5
- Structure:
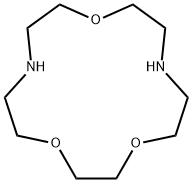
- Chemical Name:1,4,10-TRIOXA-7,13-DIAZACYCLOPENTADECANE
- CAS:31249-95-3
- MF:C10H22N2O3
- Structure:
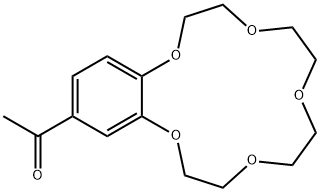
- Chemical Name:4'-ACETYLBENZO-15-CROWN 5-ETHER
- CAS:41757-95-3
- MF:C16H22O6
- Structure:
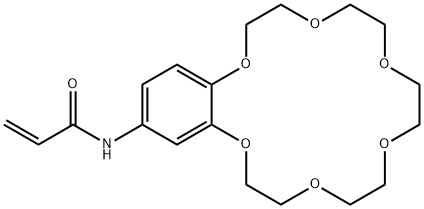
- Chemical Name:4-ACRYLAMIDOBENZO-18-CROWN-6, 98
- CAS:68865-32-7
- MF:C19H27NO7
- Structure:
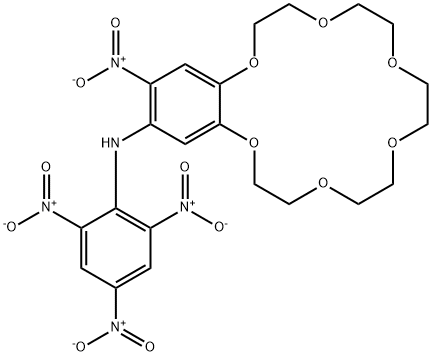
- Chemical Name:4'-NITRO-5'-(PICRYLAMINO)BENZO-18-CROWN-6
- CAS:74305-50-3
- MF:C22H25N5O14
- Structure:
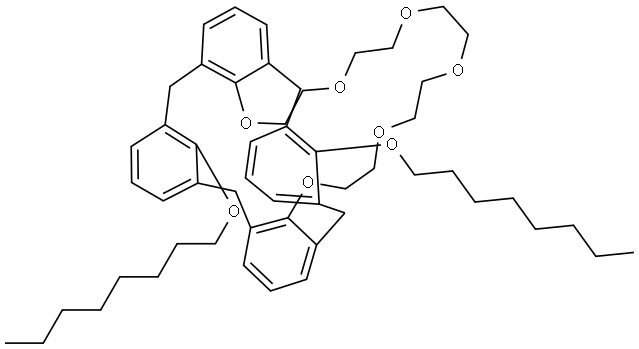
- Chemical Name:1,3-DIOCTYLOXYCALIX[4!ARENECROWN-6, 97
- CAS:161282-97-9
- MF:C54H74O8
- Structure:
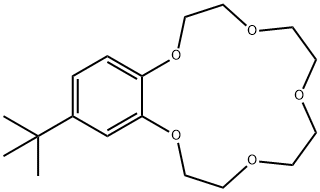
- Chemical Name:4-TERT-BUTYLBENZO-15-CROWN-5
- CAS:15196-73-3
- MF:C18H28O5
- Structure:
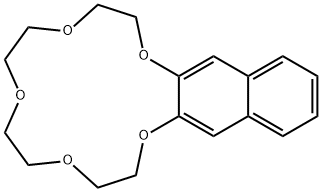
- Chemical Name:2,3-NAPHTHO-15-CROWN-5
- CAS:17454-47-6
- MF:C18H22O5
- Structure:
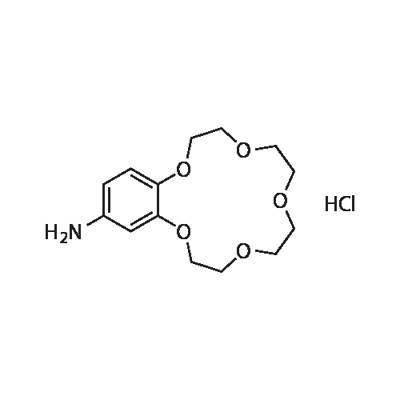
- Chemical Name:4-Aminobenzo-15-crown-5 hydrochloride
- CAS:111076-66-5
- MF:C14H21NO5.ClH
- Structure:
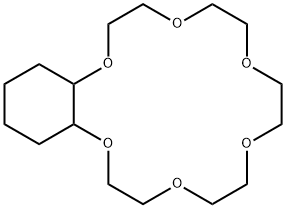
- Chemical Name:CYCLOHEXANO-18-CROWN-6
- CAS:17454-53-4
- MF:C16H30O6
- Structure:
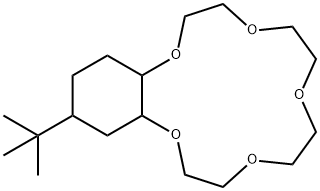
- Chemical Name:4-T-BUTYLCYCLOHEXANO-15-CROWN-5
- CAS:17454-49-8
- MF:C18H34O5
- Structure:
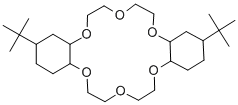
- Chemical Name:4',4''(5'')-DI-TERT-BUTYLDICYCLOHEXANO-18-CROWN-6
- CAS:28801-57-2
- MF:C28H52O6
- Structure:
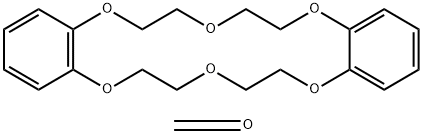
- Chemical Name:POLY(DIBENZO-18-CROWN-6)
- CAS:53660-42-7
- MF:C21H26O7
- Structure:
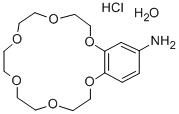
- Chemical Name:4-AMINOBENZO-18-CROWN-6 SESQUIHYDRATE HYDROCHLORIDE, 99
- CAS:205504-06-9
- MF:C16H25NO6.ClH.H2O
- Structure:
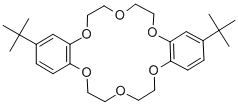
- Chemical Name:4',4''(5'')-Di-tert-butyldibenzo-18-crown-6
- CAS:29471-17-8
- MF:C28H40O6
- Structure:
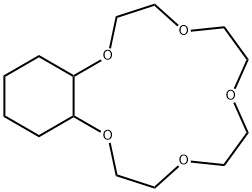
- Chemical Name:CYCLOHEXANO-15-CROWN-5
- CAS:17454-48-7
- MF:C14H26O5
- Structure:
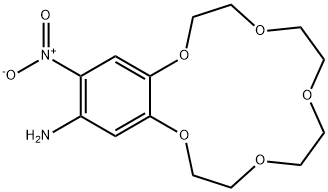
- Chemical Name:4'-AMINO-5'-NITROBENZO-15-CROWN-5
- CAS:77001-50-4
- MF:C14H20N2O7