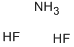white scales or flakes, also referred to asammonium hydrogen fluoride, anunonium difluoride and anunonium acid fluoride.
Orthorhombic or tetragonal crystals; etches glass; deliquescent; density 1.50 g/cm3; refractive index 1.390; melts at 125.6°C; very soluble in water; slightly soluble in alcohol.
In manufacture of Mg and Mg alloys; in brightening of Al; for purifying and cleansing various parts of beer-dispensing apparatus, tubes, etc., sterilizing dairy and other food equipment; in glass and porcelain industries; as mordant for aluminum; as a "sour" in laundering cloth. In lab production of HF.
Ammonium hydrogen fluoride is used as a chemical reagent in analysis and an antiseptic. It is also used in ceramics, electroplating, component of glass etchants as well as food processing equipment disinfectant. It acts as an intermediate in the production of hydrofluoric acid from hexafluorosilicic acid.
Ammonium bifluoride solution is the white crystalline solid dissolved in water. Ammonium hydrogen difluoride is corrosive to metals and tissue. Ammonium hydrogen difluoride is used in ceramics.
Dissolves in water and forms a weak solution of hydrofluoric acid.
AMMONIUM BIFLUORIDE reacts violently with bases. In presence of moisture will corrode glass, cement, and most metals. Flammable hydrogen gas may collect in enclosed spaces. Do not use steel, nickel, or aluminum containers [USCG, 1999].
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Some are oxidizers and may ignite combustibles (wood, paper, oil, clothing, etc.). Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated.
Flammability and Explosibility
Non flammable
Caustic poison and strong irritant by all routes. See also HYDROFLUORIC ACID. When heated to decomposition it emits very toxic fumes of F-, NO,, and NH3.
It is used as a sterilizer, in dairy and
brewery operations; in the ceramic, glass, and electroplating
industries; as a laundry sour.
Administer oxygen if breathing is difficult. Remove and isolate
contaminated clothing and shoes. In case of contact with
substance, immediately flush skin or eyes with running
water for at least 20 minutes. For minor skin contact, avoid
spreading material on unaffected skin. Keep victim warm
and quiet. Effects of exposure (inhalation, ingestion or skin
contact) to substance may be delayed. Ensure that medical
personnel are aware of the material(s) involved and take precautions
to protect themselves. Medical observation is
recommended for 24 to 48 hours after breathing overexposure,
as pulmonary edema may be delayed. As first aid for
pulmonary edema, a doctor or authorized paramedic may
consider administering a drug or other inhalation therapy.
Incompatible with oxidizers (chlorates,
nitrates, peroxides, permanganates, perchlorates, chlorine,
bromine, fluorine, etc.); contact may cause fires or explosions.
Keep away from alkaline materials, strong bases,
strong acids, oxoacids, epoxides. In the presence of moisture
corrodes concrete, metals, glass.
May be buried in a specially
designated chemical landfill. Aqueous wastes may be reacted with an excess of lime followed by lagooning and
either recovery or land disposal of the separated calcium
fluoride.