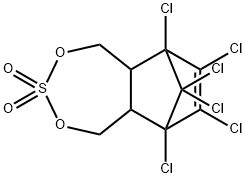
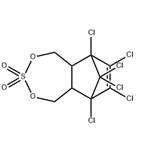
Endosulfan Sulfate is a pesticide used in the control and protection of plant and vegetation.
Water solubility - <1 mg/L at 72°F.
ENDOSULFAN SULFATE is an organochlorine and cyclodiene. ENDOSULFAN SULFATE is also a sulfite ester. Corrosive to iron. Hydrolyzed by aqueous alkali or acid, generating sulfur dioxide. Incompatible with strong oxidizing and reducing agents. Also incompatible with many amines, nitrides, azo/diazo compounds, alkali metals, and epoxides. As an ester, ENDOSULFAN SULFATE will hydrolyze to form sulfur dioxide and diol; reaction is more rapid under basic conditions.
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Soil. A mixed culture of soil microorganisms biodegraded endosulfan sulfate to
endosulfan ether, endosulfan-a-hydroxy ether and endosulfan lactone (Verschueren, 1983).
Indigenous microorganisms obtained from a sandy loam degraded endosulfan sulfate (a
metabolite of a- and b-endosulfan) to endosulfan diol. This diol was converted to endosulfan
a-hydroxy ether and trace amounts of endosulfan ether and both were degraded to
endosulfan lactone (Miles and Moy, 1979). Using settled domestic wastewater inoculum,
endosulfan sulfate (5 and 10 mg/L) did not degrade after 28 days of incubation at 25°C
(Tabak et al., 1981).
Plant. In tobacco leaves, endosulfan sulfate was converted to a-endosulfan which
subsequently hydrolyzed into endosulfandiol (Chopra and Mahfouz, 1977).