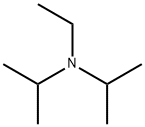
N,N-Diisopropylethylamine is also known as Hunig's base and abbreviated as DIPEA or DIEA, It is a sterically hindered amine and an organic compound. The colourless liquid was named as Hung’s base after Siegfried Hunig, a German chemist. It is noteworthy that the compound is commercially available.
In organic chemistry, N,N-Diisopropylethylamine is used as a base. Since the nitrogen centre is isolated by an ethyl group and the two isopropyl groups, It can bind to protons. The compound is, therefore, a base similar to 2,2,6,6-tetramethylpiperidine, but a poor nucleophile, a blend of properties that makes it valuable as an organic reagent.
DIPEA exhibits violent reaction as well as flammability with nitrates, oxidizing agents, and peroxides.
It can also react very exothermically and possibility of spitting with halogens and strong acids. In an alkaline environment, the compound is likely to react violently. In addition, the compound can form toxic products such as n-nitrosamines when combined with nitrous acid as well as oxygen, nitrosating agents, and nitrates.
Under normal conditions (temperature and pressure), DIPEA is very stable. However, it is soluble in most organic solvents.
N,N-Diisopropylethylamine is utilized as a base in the palladium(0)catalysed alkoxycarbonylation of both allyl acetates and phosphates. It is used as a neutralizer of the produced phosphoric acid. Notably, the alkyl ester cannot be produced without DIPEA.
When combined with boryl triflates, N,N-Diisopropylethylamine is used in the enolate synthesis of ketones for application in directed cross-adol reactions.
DIPEA is applied as a proton scavenger in organic synthesis. Since the compound is a sterically hindered amine, it lacks quaternization; therefore, making it a perfect choice of a base for use with extremely reactive alkylating agents. DIPEA is specifically useful as a base in the protection of alcohols as substituted ethers in the field of protecting group chemistry.
In the synthesis of peptides, the compound is also used in the coupling of amino acids. The steric nature and basicity of DIPEA during the coupling reaction affects the degree of racemization.
Diisopropylethylamine (DIPEA) is a clear, colorless to light yellow liquid, insoluble in water and easily soluble in acetone and other organic solvents.
N,N-Diisopropylethylamine is used as a base in organic reactions. Used in the preparation of (-)Gambierol a marine polycyclic ether toxin. It is also used in the synthesis of potent inhibitors of human brain memapsin, a key effector in the progression of Alzheimer’s disease.
N,N-Diisopropylethylamine is an important pesticide and pharmaceutical intermediate, which can be used to synthesize anesthetics and herbicides, and can also be used as a sterically hindered amine to participate in various catalytic reactions.
N,N-Diisopropylethylamine is used as a non-nucleophilic base in organic synthesis. It is prepared by the alkylation of diisopropylamine with diethyl sulphate. DIPEA can then be purified through distillation from potassium hydroxide if necessary.
ChEBI: N,N-Diisopropylethylamine (DIPEA) is a tertiary amino compound.
N-Ethyldiisopropylamine (EDIA) reacts with monoactivated Michael acceptors to afford symmetrical sulfones.
Flammability and Explosibility
Highly flammable
Distil the amine from ninhydrin, then from KOH [Dryland & Sheppard, J Chem Soc, Faraday Trans 1 125 1986]. It is a strong base and should be stored in the absence of carbon dioxide. [Hünig & Kiessel Chem Ber 91 380, 387 1958, Wotiz et al. J Org Chem 24 1202 1959, Beilstein 4 IV 551.]