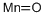Green cubic crystal; refractive index 2.16; density 5.37 g/cm3 at 23°C; Moh’s hardness 5.5; melts at 1945°C; insoluble in water.
Manganese(II) oxide is used in the fertilizer industry as a source of manganese in fertilizers; in feedstuff formulations; and as an intermediate in the production of several manganese compounds.
Manganese(II) oxide is obtained commercially from manganese(IV) oxide (manganese dioxide) by the reduction with hydrogen, carbon monoxide or methane at elevated temperatures (>800°C):
MnO2 + CO → MnO + CO2
MnO2 + H2 → MnO + H2O
The oxide also can be made by thermal decomposition of manganese(II) carbonate or manganese(II) oxalate in the absence of air:
MnCO3 → MnO + CO2
Also, careful dehydration of manganese(II) hydroxide, Mn(OH)2, under controlled conditions in the absence of air yields MnO.
Manganese(II) oxide is the lowest oxide of manganese and it is purely a basic oxide. It reacts with acids to form their manganese(II) salts:
MnO + H2SO4 → MnSO4 + H2O
MnO + 2HCl → MnCl2 + H2O
The compound also is oxidized by air or oxygen to higher oxides of manganese. When heated cautiously in air, the product is manganese sesquioxide or manganese(III) oxide:
4MnO + O2 → 2Mn2O3
grass green powder(s); cub; enthalpy of fusion 54.40kJ/mol; produced from MnO2 ores by roasting in a reducing atmospheric, e.g., methane; used in textile printing, in ceramics, in paints [HAW93] [CRC10]
The main use of manganese dioxide is in dry-cell batteries, such as the alkaline battery and the zinc-carbon battery. It is useful as a pigment, and as a precursor to other manganese compounds. It is used as a reagent in organic synthesis, for instance, for the oxidation of allylic alcohols and arylmethyl alcohols. It is also employed in various industrial purposes such as adhesives and sealant chemicals, lubricants and lubricant additives, fertilizers, oxidizing/reducing agents, plating agents and surface treating agents, processing aids, air care products, paints and coatings.
Manganous monoxide (MnO) is used for textile printing, ceramics, paints, colored (green)
glass, bleaching, and fertilizers. It is also used as dietary supplement and reagent in analytical
chemistry.
Flammability and Explosibility
Not classified
Moderately toxic by
intratracheal and subcutaneous routes.
Violent reaction with hydrogen peroxide,
Ca(OCl)2, F2, H202. See also
MANGANESE COMPOUNDS.