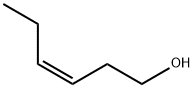
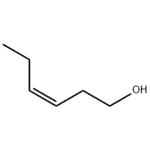
Leaf alcohol exists as a liquid at room temperature with a characteristic odor of green leaves. It is found in green tea, violet leaf oil, and many types of leaves, herbs, and grasses. Leaf alcohol finds applications in perfumery as floral fragrance. Leaf alcohol is also investigated for its antidiabetic activity.
3-Hexen-1-ol has an intense, grassy-green odor, not as strong as the corresponding aldehyde, and a characteristic her�baceous, leafy odor on dilution.
cis-3-Hexen-l-ol has an intense, green odor, not as strong as the
corresponding aldehyde and a characteristic herbaceous, leafy
odor on dilution. This substance can be obtained through extraction
from various essential oils and purified by reacting it to the corresponding phthalate or allophanate; it was synthesized by Ruzicka
and Schinz, who also clarified its chemical structure; Stoll and
Rouve reported on the most significant differences between the
natural and the synthetic products.
Leaf Alcohol is a colorless
liquid with the characteristic odor of freshly cut grass. In small quantities,
leaf alcohol occurs in the green parts of nearly all plants. The volatile flavor
constituents of green tea contain up to 30%.
A stereospecific synthesis of (Z)-3-hexen-1-ol starts with the ethylation of
sodium acetylide to 1-butyne, which is reacted with ethylene oxide to give
3-hexyn-1-ol. Selective hydrogenation of the triple bond in the presence of
palladium catalysts yields (Z)-3-hexen-1-ol. Biotechnological processes have
been developed for its synthesis as a natural flavor compound, for example.
Leaf alcohol is used to obtain natural green top notes in perfumes and flavors. In
addition, it is the starting material for the synthesis of (2E,6Z)-2,6-nonadien-l-ol
and (2E,6Z)-2,6-nonadien-l-al.
Main constituent of the oil distilled from the infusion of fermented tea leaves. Reported found as the cor�responding ester of phenylacetic acid in the oil of Japanese mint (Mentha arvensis); the volatile oil of Thea chinensis contains
approximately 26 to 35% 3-hexen-1-ol, whereas larger amounts are reported in the oils of Morus bombysic, Robinia pseudacacia and
Raphanus sativus. Probably occurring also in several green leaves and herbs; reported found in the fruit juices of raspberry, grape�fruit and others. Also reported in over 200 foods including apple, apricot, banana, citrus peel oils and juices, berries, guava, mango,
grapes, pineapple, cabbage, kohlrabi, celery, cucumber, lettuce, leek, peas, sauerkraut, tomato, ginger, peppermint oil, coconut oil,
spearmint oil, mustard, parsley, breads, butter, fish, fish oil, cognac, brandy, cider, sherry, grape wines, tea, soybeans, avocado,
olive, passion fruit, plum, rose apple, Malay apple, water apple (Syzigium spp.), beans, marjoram, starfruit, broccoli, pear and apple
brandies, figs, brussels sprouts, radish, prickly pear, litchi, dill, lovage, pumpkin, corn oil, malt, laurel, kiwifruit and other sources
cis-3-Hexen-1-ol is a naturally occuring compound that has the smell of freshly cut grass and is used to obtain a тАЬgreenтАЭ taste/smell in certain flavours and fragrances.
ChEBI: A primary alcohol that consists of (3Z)-hex-3-ene substituted by a hydroxy group at position 1.
By the reaction of butyne-1 with ethylene oxide and subsequent selective reduction to the eis isomer (Bedoukian, 1967).
Taste characteristics at 30 ppm: fresh, green, raw fruity with a pungent depth
cis-3-Hexen-1-ol is one of the key volatile constituents of green leaf volatiles(GLV) that can act as an attractant to various insects. It is emitted by green plants when they are physically damaged.
Flammability and Explosibility
Flammable
Extracted from various essential oils and purified by reacting it to the corresponding phthalate or allophanate; it was
synthesized by Ruzi-ka and Schinz, who also clarified its chemical structure; Stoll and Rouve reported on the most significant differ�ences between the natural and the synthetic products (Burdock, 1995)
[1] NPCS Board of Consultants & Engineers, Industrial Alcohol of Technology Handbook, 2010
[2] A. Shirwaikar, K. Rajendran and C. Kumar, Oral Antidiabetic Activity of Annona squamosa Leaf Alcohol Extract in NIDDM Rats, Pharmaceutical Biology, 2004, vol. 42, 30-35