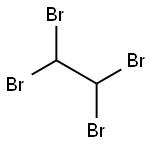
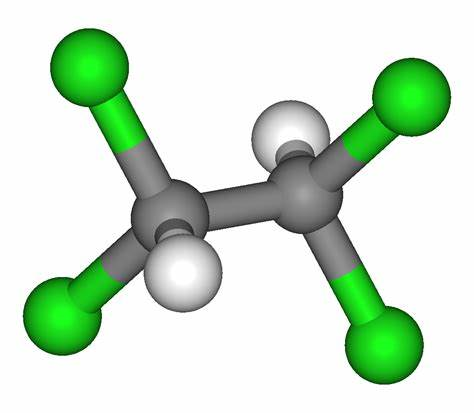
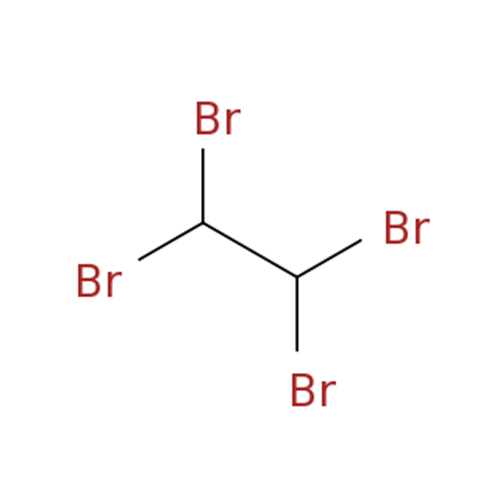
1,1,2,2-Tetrabromoethane: Properties, Production process and Uses
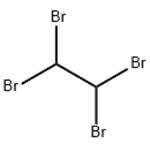
1,1,2,2-Tetrabromoethane, also known as acetylene tetrabromide, sym-tetrabromoethane,
tetrabromoacetylene and s-tetrabromoethane, is a derivative of acetylene and bromine. Molecular formula: C2H2Br4, molecular weight: 345.653.
Properties of 1,1,2,2-tetrabromoethane
1,1,2,2-Tetrabromoethane is a colorless to light yellow, transparent and oily liquid
with a boiling point of 243.5°C. The industrial product is a light yellow or amber
liquid with an odor of camphor and iodoform. It is miscible with organic solvents
such as alcohol, ether, chloroform, carbon tetrachloride, aniline and glacial acetic
acid. 1,1,2,2-Tetrabromoethane is slightly soluble in water with a solubility of
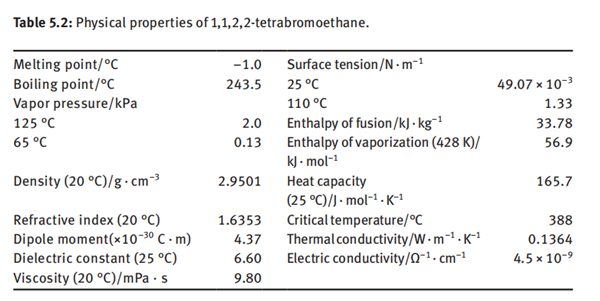
0.065% at 30°C. The physical properties of 1,1,2,2-tetrabromoethane are listed in
Table 5.2.
1,1,2,2-Tetrabromoethane reacts with a strong base to generate hydrogen bromide. It is stable at room temperature, yet it will be decomposed upon heating to above 240 °C, resulting in the release of bromine and hydrogen bromide.
Process for manufacture of 1,1,2,2-tetrabromoethane
1,1,2,2-Tetrabromoethane is obtained by addition reaction of acetylene with bromine. The reaction proceeds easily in the absence of a catalyst. In order to avoid the occurrence of substitution reactions and other side reactions, the reaction is usually
carried out at a lower temperature (<60°C). The reaction equation is as follows:
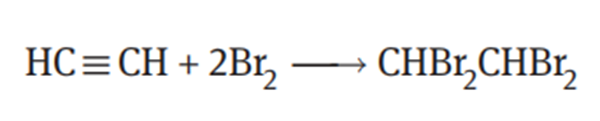
Bromine was added to the glass-lined reactor and a small amount of water was added to block the bromine liquid surface to prevent it from volatilization. Then, acetylene was slowly introduced into the reactor with a pressure of 0.02–0.05 MPa. The reaction was carried out at a temperature of 50–60°C for 5–7 h. During the reaction, the liquid gradually turned yellow. The reaction solution was washed with a dilute alkali solution, and then allowed to stand for separating into two layers. The lower organic phase was collected, washed with water until neutral to obtain 1,1,2,2-tetrabromoethane.
The 1,1,2,2-tetrabromoethane synthesis reaction is performed in two steps. First, acetylene reacts with bromine to form dibromoethylene, and then the resultant dibromoethylene further reacts with bromine to give 1,1,2,2-tetrabromoethane:
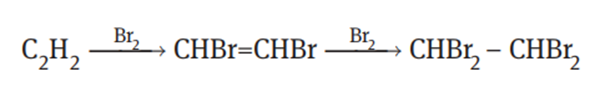
Both alkynes and alkenes can undergo an electrophilic addition reaction. Since there
are two π bonds in the acetylene triple bond, they can generally be added with two
molecular reagents. Apparently, it seems that the alkyne is easier to proceed electrophilic addition than the alkene, but in fact, bromine preferentially undergoes an
addition reaction with the alkene to form tetrabromoethane. Therefore, side reactions
are not easy to occur. However, if the acetylene is largely excessive, by-products such
as dibromoethylene and tribromoethane are generated.
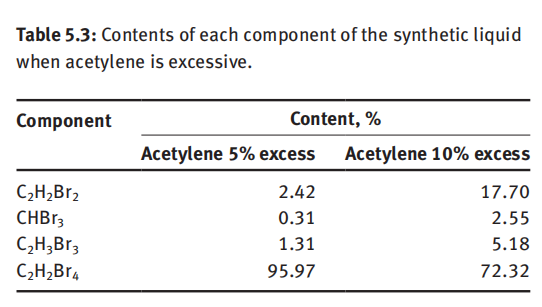
The composition of reaction solution produced from the synthesis tower is relatively complicated. If the actual ratio of bromine and acetylene is closer to the stoichiometric ratio during the reaction, the impurity content in the reaction solution is less, and content of 1,1,2,2-tetrabromoethane is higher. In practice, acetylene is only slightly excessive, for example, 1–5% excess. If the excess of acetylene is more than 10%, the content of dibromoethylene and tribromoethane in the reaction solution is significant, as shown in Table 5.3.
Increasing the illumination (visible or ultraviolet light) can promote the addition reaction of acetylene and bromine, shorten the reaction time, increase the content of 1,1,2,2-tetrabromoethane, and reduce the content of by-products in the reaction solution.
A pH of 5 to 7 is required for the finished product of 1,1,2,2-tetrabromoethane, which is close to neutral. However, hydrobromic acid is present throughout the production process, resulting in an unacceptable acidity index. Usually in industry, solid sodium carbonate is added to the acidic reaction solution to neutralize the acid produced during the synthesis. The base added here must be a solid rather than an aqueous solution; otherwise, a side reaction of elimination will occur.
Uses of 1,1,2,2-tetrabromoethane
1,1,2,2-Tetrabromoethane is used as a cocatalyst for the oxidation process in the production of terephthalic acid. It is also used as a flame retardant in plastic products such as expandable polystyrene foam. Furthermore, it is an intermediate for synthesis of quaternary ammonium salts, fuel, refrigerant, disinfectant and pesticides. Moreover, it is applied as a mineral flotation agent and oil field killing fluid.
);You may like
See also
Lastest Price from 1,1,2,2-Tetrabromoethane manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 79-27-6
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $20.00-5.00/kg2023-12-11
- CAS:
- 79-27-6
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons