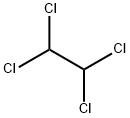
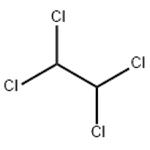
1,1,2,2-Tetrachloroethane: Properties, Production process and Uses
1,1,2,2-Tetrachloroethane, also known as s-tetrachloroethane, acetylene tetrachloride,
tetrachloroethane and sym-tetrachloroethane, is a derivative of acetylene and chlorine. Molecular formula: C2H2Cl4, molecular weight:
167.849.
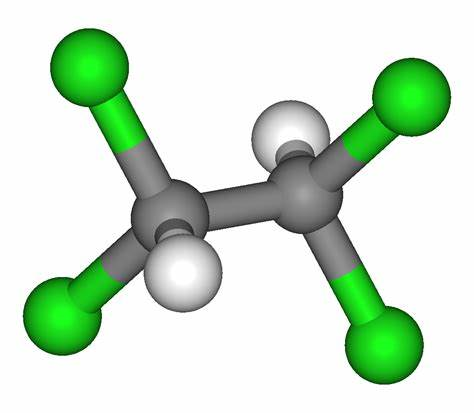
Properties of 1,1,2,2-tetrachloroethane
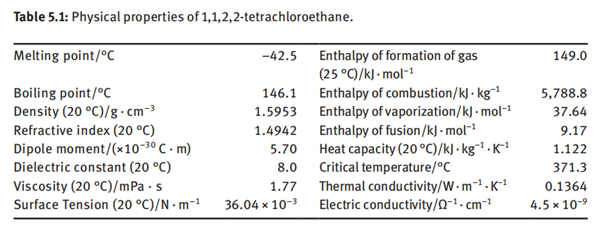
1,1,2,2-Tetrachloroethane is a non-flammable, non-explosive, colorless and transparent liquid with a strong odor similar to chloroform. The boiling point is 146.3°C, and the melting point is −42.5°C. It can be miscible with most organic solvents such as alcohol, ether, petroleum ether, halogenated hydrocarbon and carbon disulfide. It has the strongest dissolving power among chlorinated hydrocarbons. It can dissolve various organic substances including lipid, wax, asphalt, coal tar, camphor, rubber, dye, ethyl cellulose, nitrocellulose and polyvinyl chloride as well as inorganic substances such as sulfur, phosphorus, halogen and sodium sulfite. At 120°C, 100 g of 1,1,2,2-tetrachloroethane can dissolve 100 g of sulfur. It is slightly soluble in water. The solubility in water at 25°C is 0.29% (mass fraction), while the solubility of water in 1,1,2,2-tetrachloroethane is 0.13%. It forms an azeotrope with water, having an azeotropic point of 93.2°C and an azeotropic composition: 1,1,2,2-tetrachloroethane of 68.9% and water of 31.1%. The physical properties of 1,1,2,2-tetrachloroethane are listed in Table 5.1.
The toxicity of this product is similar to chloroform, and the allowable concentration in air at working place is 5 × 10−6 in China. The impure product of 1,1,2,2-tetrachloroethane is easily decomposed into hydrogen chloride and trichloroethylene, while pure product is relatively stable in the absence of air, moisture and light, but are easily decomposed into trichloroethylene in the presence of alkali. When 1,1,2,2-tetrachloroethane is in contact with air, it slowly removes hydrogen chloride to form trichloroethylene and traces of phosgene. In the presence of moisture, it gradually decomposes and releases hydrogen chloride.
1,1,2,2-Tetrachloroethane is converted into dichloroacetyl chloride by irradiation with
ultraviolet light in the presence of air or oxygen. It does not react with chlorine at room
temperature, but chlorination occurs under ultraviolet light, resulting in the generation of hexachloroethane. 1,1,2,2-Tetrachloroethane is reduced to 1,2-dichloroethylene
by treating with a metal such as iron, aluminum or zinc in the presence of boiling
water or steam. It generates a highly explosive dichloroacetylene upon heating in the
presence of a strong base.
Process for manufacture of 1,1,2,2-tetrachloroethane
1,1,2,2-Tetrachloroethane is prepared by addition reaction of acetylene with chlorine:
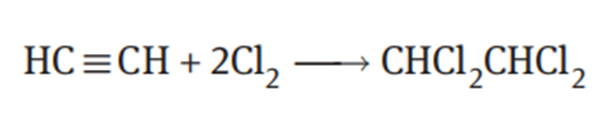
Since direct reaction of the gaseous acetylene with chlorine may cause an explosion, the reaction is carried out using 1,1,2,2-tetrachloroethane itself as the solvent. The catalyst is stibium pentachloride or ferric chloride. When the ferric chloride catalyst is used, dry acetylene and chlorine are continuously introduced into the tetrachloroethane which is kept in reflux under reduced pressure. The heat of reaction is absorbed by evaporation of the tetrachloroethane and removed by the reflux condenser. The yield of 1,1,2,2-tetrachloroethane is 97% based on acetylene.
Uses of 1,1,2,2-tetrachloroethane
1,1,2,2-Tetrachloroethane is used as an intermediate for trichloroethylene, tetrachloroethylene, pentachloroethane and hexachloroethane. As a nonflammable solvent, it can be used to dissolve shellac, resins, waxes, and others but it is highly toxic and prone to hydrolysis, which limits its application. In addition, it is also used in the production of metal detergents, paint removers, insecticides, herbicides, alcohol denaturants and the like.
);You may like
See also
Lastest Price from 1,1,2,2-Tetrachloroethane manufacturers
US $50.00-1.00/KG2024-03-25
- CAS:
- 79-34-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $0.00/KG2023-06-29
- CAS:
- 79-34-5
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month