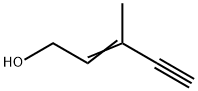
3-Methyl-2-penten-4-yn-1-ol: Properties and Production process
Properties of 3-methyl-2-penten-4-yn-1-ol
The cis isomer is an oily liquid with a boiling point of 65°C (1.253 kPa) and a refractive index of 1.4820 (20°C). The trans isomer is also an oily liquid with a boiling point of 73°C (1.253 kPa) and a refractive index of 1.4934 (20°C). Both isomers are heatsensitive substances and easy to be polymerized because of double bonds and triple bonds in their molecular structures.
Production process of 3-methyl-2-penten-4-yn-1-ol
In the presence of metal calcium, methyl vinyl ketone reacts with acetylene to produce iso-C6 alcohol (3-methyl-1-penten-4-yn-3-ol) which can be converted to C6 alcohol via a rearrangement reaction catalyzed by sulfuric acid:
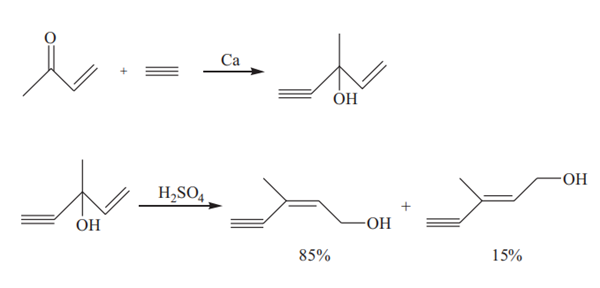
First, liquid ammonia is added to the reaction vessel, at a temperature below −40°C. Metal calcium is added and simultaneously acetylene is introduced into the liquid ammonia which is under stirring. Calcium acetylide is formed and dispersed in the liquid ammonia solvent. The reaction mixture is cooled to –60°C, then methyl vinyl ketone is added while acetylene is still bubbled therethrough at a moderate rate. The temperature of the reaction mixture is controlled at –55°C for 25 minutes. The temperature was raised to –5°C within 90 minutes in order to evaporate the ammonia. Equal amounts of diethyl ether and water are added followed by the addition of a 50% aqueous solution of acetic acid until neutral. During the additions, the temperature is controlled below 15°C. The mixture is allowed to stand for separating into two layers: a water layer and an organic layer. The water layer is extracted twice with diethyl ether. The combined organic layers are washed with aqueous solution of sodium bicarbonate, dried over anhydrous magnesium sulphate and then distilled after recovery of diethyl ether. The yield of iso-C6 alcohol is 71% based on methyl vinyl ketone.
115 g of iso-C6 alcohol is dissolved in 250 ml solvent (chloroform or isopropyl ether) under stirring, and 300 mL of dilute sulfuric acid (21.5%) is added to the solution. The reaction is carried out at a temperature of 55°C for 2h. After the reaction, the reaction mixture is cooled and allowed to stand for separating into two layers. The organic layer is washed twice with aqueous solution of sodium bicarbonate (100 mL×2), dried over anhydrous magnesium sulphate and then distilled after recovery of the solvent. The yield of C6 alcohol is 86.6% based on iso-C6 alcohol and the content of cis isomer in the C6 alcohol is 85.1%.
Sulfuric acid-catalyzed allyl rearrangement of 3-methyl-1-penten-4-yn-3-ol produces an isomeric mixture of (Z)- and (E)-3-methyl-2-penten-4-yn-1-ol. The cis isomer can be cyclized under certain conditions to form 2,3-dimethylfuran with a large exotherm. Both the isomers are heat-sensitive compounds and tend to polymerize when heated.
Recently, acidic ionic liquids are used as catalysts to achieve allylic rearrangement
of 3-methyl-1-pentene-4-yn-3-ol. For example, [HNMP]HSO4, an acidic ionic liquid
formed by the reaction of NMP and sulfuric acid in a ratio of 1:1, is very effective with
an increased proportion of cis isomer to 88% after reaction at 55°C for 6h. The ionic
liquid is not volatile and can be easily separated from the reaction products by distillation. The ionic liquid can be recycled and there is no significant reduction in catalytic
activity and selectivity after four cycles. However, the performance of the catalyst is
significantly reduced when it is reused more than 4 times. This is probably because
the ionic liquid is contaminated. The performance of the ionic liquid can be recovered
by washing with petroleum ether or dichloromethane to remove the contaminants.
In industry, the reaction products of the allyl rearrangement usually contain cis isomer (78–85%), trans isomer (10–15%), light fractions (3–5%) and other impurities (2–4%). The trans isomer is always present in the reaction mixture. Optimization of reaction conditions such as temperature, solvent type and acid concentration cannot completely eliminate the formation of trans isomer. The cis isomer is an important intermediate for the manufacture of vitamin A. The trans isomer cannot be used to produce vitamin A. The cis and trans isomers can be separated from each other by fractional distillation.
Since the boiling points of cis and trans C6 alcohols are not much different, so a large number of plates are required to separate each other by fractional distillation. An increase in the number of plates will increase the pressure drop of the tower, leading to the increase in the boiling point of the C6 alcohols. Therefore, a higher operating temperature is required in the fractional distillation. However, C6 alcohols are heat-sensitive compounds and easy to polymerize or decompose because of acetylenic and ethylenic bonds in their molecular structures.
The use of a falling film reboiler and steam distillation reduces the operating temperature in the fractional distillation, reduces the decomposition and polymerization
of C6 alcohols and improves the safety production factor.
Figure 4.8 displays the process flow diagram for continuous steam distillation of 3-methyl-2-penten-4-yn-1-ol. The chloroform solution of crude C6 alcohols was preheated by feed preheater (2) and then introduced into the solvent removal tower (3) for fractional distillation. The solvent separated at the top of the tower is condensed by a condenser (4) and collected in the solvent receiving tank (7). The bottom product of the solvent removal tower (3) is collected in the intermediate tank (9) and preheated by another preheater (10) before being fed to the middle section of the product distillation tower (11). An appropriate amount of steam is introduced into the top of the falling film reboiler (16), and steam distillation was performed under negative pressure. Wire web packings with high efficiency and low pressure drop are applied in the distillation tower. The vapor distilled from the top of the product distillation tower (11) is condensed by condenser (12) to obtain a mixture of light fractions and water, and some of them are withdrawn as the overhead product by controlling a certain reflux ratio. Final product of cis-C6 alcohol (15) is withdrawn as a liquid side stream in the rectifying section of the tower. The bottom product (19) is withdrawn and cooled by a cooler (18) to obtain trans isomer which contains a small amount of oligomers. The yield of cis-C6 alcohol is over 96% and the purity is more than 99%.