5-Sulfosalicylic acid dihydrate: properties, applications and safety
General Description
5-Sulfosalicylic acid dihydrate is a white to yellowish-white powder with slightly soluble properties. It serves as a weak acid and finds applications as a pH buffering agent, indicator, and antioxidant. It is used in protein electrophoresis, metal detection, organic synthesis, and industrial processes. The compound has solubility in water and alcohol, a pH range of 2.5 to 4.0, and a melting point of 180°C to 182°C. Safety precautions should be taken due to its irritant and toxic nature. Proper handling, protective equipment, and storage away from flames are necessary.

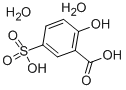
Figure 1. 5-Sulfosalicylic acid dihydrate
Properties
5-Sulfosalicylic acid dihydrate is a white to yellowish-white powder with slightly soluble properties in water and solubility in both alcohol and hot water. With a molecular formula of C7H6O6S and a molecular weight of 218.20, it serves as a weak acid with multiple applications in the chemical industry. This compound acts as a pH buffering agent, indicator, and antioxidant. Notably, its solubility characteristics enable it to effectively perform its functions in various aqueous and alcoholic solutions. Additionally, 5-sulfosalicylic acid dihydrate exhibits a pH value ranging from 2.5 to 4.0 and possesses a melting point in the range of 180°C to 182°C. Its boiling point falls between 330°C and 350°C, while its specific gravity measures at 1.82. The flash point of this compound is determined to be 202°C, and its decomposition point ranges from 280°C to 300°C. These properties contribute to its versatility and usefulness in chemical processes and applications. 1
Applications
5-Sulfosalicylic acid dihydrate has various applications in different fields. One of its primary uses is as a fixing solution in protein electrophoresis, which is a technique used to separate and analyze proteins based on their size and charge. This reagent helps stabilize and immobilize proteins during the process, allowing for accurate analysis. In industry, 5-sulfosalicylic acid dihydrate finds application as a metal chelating agent. It forms complexes with metal ions, helping to remove them from solutions or bind to them for certain chemical processes. Additionally, it is utilized in the preparation of surface-active agents, which are compounds that alter the properties of surfaces, organic catalysts, and grease additives. The reagent is also used in the detection of metals in solutions and solid state samples. Researchers have reported its effectiveness in identifying and quantifying metal ions in various substances. Furthermore, a reversed-phase HPLC (High-Performance Liquid Chromatography) method has been developed using 5-sulfosalicylic acid for detecting short chain coenzyme A esters in tissue samples. Another application of 5-sulfosalicylic acid dihydrate is in protein precipitation of plasma samples before HPLC analysis. This technique is used to remove unwanted proteins from plasma samples, enabling more accurate and specific analysis of target substances such as mitoxantrone and 6-mercaptopurine. Overall, 5-sulfosalicylic acid dihydrate plays a crucial role in protein analysis, metal detection, organic synthesis, and various industrial processes. Its properties make it a valuable reagent in these applications, contributing to scientific research and quality control in different fields. 2, 3
Safety
5-Sulfosalicylic acid dihydrate poses certain safety risks that need careful consideration. Firstly, this compound can irritate the eyes and skin upon contact, as well as the respiratory system if inhaled. It is essential to wear appropriate protective gear like goggles, gloves, and a face shield when handling it. Secondly, 5-sulfosalicylic acid dihydrate is flammable and should be stored away from open flames or high temperatures. A cool and dry storage place is recommended to prevent fire hazards. Thirdly, this chemical is toxic if inhaled or ingested. Exposure to high concentrations can cause respiratory tract irritation, nausea, vomiting, and other health issues. If exposed, remove the source of exposure, seek fresh air immediately, and obtain medical assistance if necessary. To ensure safety, users should adopt proper safety practices, use personal protective equipment, and refer to reliable safety information sources or authorities for detailed guidance on handling and usage of 5-sulfosalicylic acid dihydrate. 4
Reference
1. PubChem. Compound Summary: 5-Sulfosalicylic acid dihydrate. National Library of Medicine, 2005.
2. Foti C, Gianguzza A, Orecchio S, Piazzese D. Protonation and complex formation of 5-sulfosalicylate in NaCl, CaCl2 and MgCl2 aqueous media. Speciation in synthetic seawater. Ann Chim, 2002, 92(5-6):551-562.
3. Demoz A, Garras A, Asiedu DK, Netteland B, Berge RK. Rapid method for the separation and detection of tissue short-chain coenzyme A esters by reversed-phase high-performance liquid chromatography. J Chromatogr B Biomed Appl, 1995, 667(1):148-152.
4. SAFETY DATA SHEET: 5-Sulfosalicylic acid dihydrate. Thermo Fisher SCIENTIFIC, 2013, Cat No.A2973.
);You may like
Related articles And Qustion
Lastest Price from 5-Sulfosalicylic acid dihydrate manufacturers

US $20.00/kg2024-04-27
- CAS:
- 5965-83-3
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 200000kg

US $0.00/KG2023-06-29
- CAS:
- 5965-83-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month