Versatile Applications and Toxicity of 5-Sulfosalicylic Acid Dihydrate in Biochemistry and Analytical Chemistry
General Description
5-Sulfosalicylic Acid Dihydrate is a versatile compound widely utilized in biochemistry and analytical chemistry. In biochemistry, 5-Sulfosalicylic Acid Dihydrate serves as a fixing solution in protein electrophoresis, aiding in the separation of proteins and finding applications in metal chelation, surface-active agents, organic catalysts, and grease additives. In analytical chemistry, 5-Sulfosalicylic Acid Dihydrate plays a crucial role in metal detection, analysis of coenzyme A esters, and protein precipitation for HPLC analysis. While its LD50 in rats exceeds 2g/kg, prolonged exposure can cause skin, eye, and respiratory irritation, emphasizing the necessity for careful handling and adherence to safety protocols.

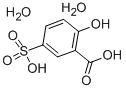
Figure 1. 5-Sulfosalicylic acid dihydrate
Versatile applications in biochemistry and analytical chemistry
Applications in protein electrophoresis
5-Sulfosalicylic acid dihydrate, also known as 3-hydroxy-5-sulfobenzoic acid dihydrate, is a versatile reagent that finds use in various industrial applications. This compound is primarily used as a fixing solution in protein electrophoresis, a technique commonly used in biochemistry for separating proteins based on their size and charge. It is added to the protein sample along with a buffer solution, which helps to maintain the pH and ionic strength of the solution during the separation process. The 5-sulfosalicylic acid dihydrate acts as a precipitating agent, causing the proteins to separate and form distinct bands in the gel. Apart from its use in protein electrophoresis, 5-sulfosalicylic acid dihydrate also finds use in other industrial applications. It is commonly used as a metal chelating agent, due to its ability to bind to metal ions and remove them from solutions. Additionally, this compound is used in the preparation of surface-active agents, which are used in detergents, emulsifiers, and wetting agents. It is also used as an organic catalyst and in the manufacture of grease additives. In conclusion, 5-sulfosalicylic acid dihydrate is a versatile reagent that finds use in a variety of industrial applications. Its primary use is as a fixing solution in protein electrophoresis, but it also has important roles in metal chelation, surface-active agents, organic catalysts, and grease additives. 1
Applications in analytical chemistry
5-Sulfosalicylic acid dihydrate is a versatile reagent that finds use in various applications in analytical chemistry. One of its major uses is in the detection of metals in solutions and samples derived from the solid state. This compound acts as a chelating agent, binding to metal ions and forming a complex that can be easily detected using techniques such as UV-Vis spectroscopy. Another important use of 5-sulfosalicylic acid dihydrate in analytical chemistry is in the analysis of short chain coenzyme A esters from tissue samples. A reversed-phase HPLC protocol has been developed for the detection of these compounds, which involves the use of 5-sulfosalicylic acid dihydrate as a protein precipitant to isolate the coenzyme A esters from the tissue samples. In addition, 5-sulfosalicylic acid dihydrate has also been used in protein precipitation of plasma samples before HPLC analysis for mitoxantrone and 6-mercaptopurine in plasma. This technique allows for the isolation and purification of these drugs from plasma samples, enabling their accurate quantification using HPLC. In conclusion, 5-sulfosalicylic acid dihydrate is a useful reagent in analytical chemistry, particularly in the detection of metals and short chain coenzyme A esters, as well as in protein precipitation of plasma samples for HPLC analysis. Its versatility and reliability make it a valuable tool in many analytical applications. 2
Toxicity
5-Sulfosalicylic Acid Dihydrate is a chemical compound commonly used in laboratory settings. Its toxicity is relatively low, with the LD50 (lethal dose for 50% of test subjects) in rats being greater than 2g/kg body weight. However, prolonged or high-level exposure to 5-Sulfosalicylic Acid Dihydrate can lead to irritation of the skin, eyes, and respiratory tract. Ingestion of large quantities may cause gastrointestinal discomfort such as nausea, vomiting, and diarrhea. As with any chemical substance, it is important to handle 5-Sulfosalicylic Acid Dihydrate with care, following proper safety protocols to minimize the risk of adverse health effects. 3
Reference
1. Product information: 5-Sulfosalicylic acid dihydrate. SIGMA. Product Number: S 3147.
2. Türkel N, Ozer U. Salicylic acid derivatives form stable complexes with scandium(III) ion in aqueous solution. Chem Pharm Bull (Tokyo). 2000;48(6):870-872.
3. 5-Sulfosalicylic acid dihydrate. National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 2723734.
);You may like
Related articles And Qustion
Lastest Price from 5-Sulfosalicylic acid dihydrate manufacturers

US $20.00/kg2024-04-27
- CAS:
- 5965-83-3
- Min. Order:
- 1kg
- Purity:
- 99.9%
- Supply Ability:
- 200000kg

US $0.00/KG2023-06-29
- CAS:
- 5965-83-3
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month