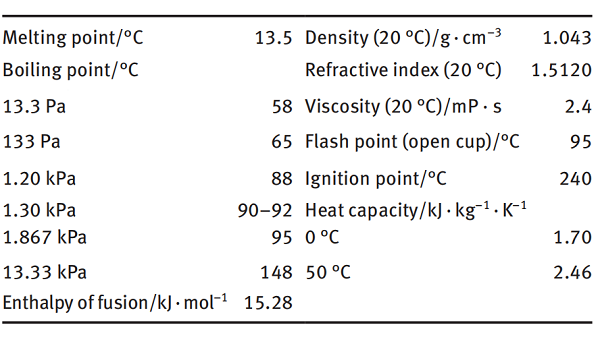
Application and preparation of N-vinyl-2-pyrrolidone
Background
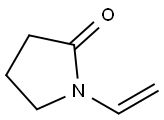
N-Vinyl-2-pyrrolidone is an organic compound with the chemical formula C6H9ON, abbreviated as NVP[1]. It is a colorless or light yellow transparent liquid with slight odor at room temperature. It is easily soluble in water and other organic solvents. It is used in radiation medicine, wood floor industry, paper or cardboard industry, packaging material, screen ink industry to improve the physical properties of products through the use of NVP.

Picture 1 N-Vinyl-2-pyrrolidone
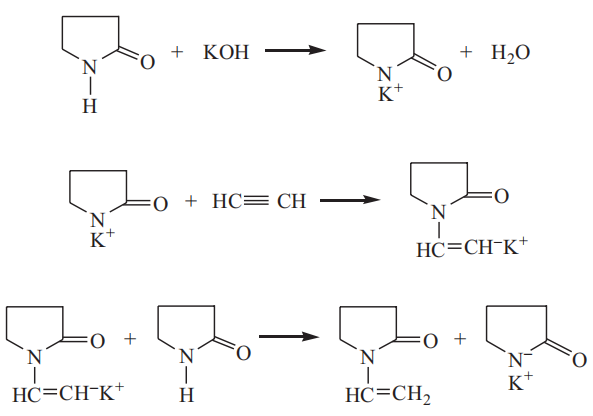
Preparation of N-vinyl‐2‐pyrrolidone
Radical suspension copolymerization of N-vinyl-2-pyrrolidone (VP) with three different cross-linkers: divinylbenzene (DVB), trimethylolpropane trimethacrylate (TRIM), and di(methacryloxymethyl) naphthalene (DMN) was used to prepare macroporous microspheres[2]. During the copolymerization, the mixture of toluene and n-dodecane as a pore-forming diluent was used. All samples were characterized in terms of particle size and distribution, nitrogen content, specific surface area total pore volume, and pore size distribution. It was found that specific surface area of the obtained beads is strongly dependent on the diluent system and the type of cross-linker and achieves value from 27 to 845 m2/g. To determine the influence of chemical structure of cross-linkers on the selectivity and polarity of the copolymers, inverse gas chromatography was applied. In addition, VP–DVB and VP–DMN copolymers were modified by sulfonation into cation-exchangers with cation exchange capacity equal 1.98 and 2.31 mmol/g, respectively.
Pulsed‐plasma polymerization of N‐vinyl‐2‐pyrrolidone
The RF plasma induced polymerization of N-vinyl-2-pyrrolidone was examined under variable duty-cycle pulsed-plasma conditions. Large-scale progressive changes in the composition of the resultant polymeric films were observed with sequential changes in the plasma duty cycle employed during polymerization, all other plasma variables held constant. The film compositional changes obtained are in the direction of increased retention of the lactam ring of the monomer in the resultant polymers as the duty cycles employed (i.e., the ratio of plasma on to plasma off times) were decreased. Particularly significant are the relatively linear polymeric structures obtained under the exceptionally low-average power deposition conditions made accessible with the pulsed plasma technique. XPS and FTIR spectroscopic examination of these latter films reveal compositions that are similar to those obtained by conventional (i.e., nonplasma) synthesis of the linear polymer. The film chemistry controllability demonstrated in the present study is achieved while maintaining the many advantages of the plasma polymerization approach for surface modifications. This work provides additional support for use of the pulsed operational mode as an effective means of film chemistry control, in particular extending the plasma polymerization technique to include synthesis of linear polymers, in lieu of the more highly crosslinked structures typically produced in conventional continuous-wave plasma polymerization processes.
Application
1. Plastic This monomer can be added to the formulation of UV-curable coatings to form a flexible and hard plastic film. Adding NVP to radiation medical formulations can improve elongation, viscosity, etc. of high-gloss and low-gloss coatings.
2. Wood enhances product performance, increases productivity and reduces the pressure on environmental pollution, which are the main reasons for the investigation of the use of scattering coatings in the furniture industry. The addition of NVP to UV-resistant coatings used in the wood floor industry can provide physical properties equivalent to wax-free floors.
3. UB and EB curing coatings used in papermaking or paperboard generally require lower viscosity and high reactivity. NVP meets these criteria by improving flow and gauge levels, while maintaining good cure characteristics.
Cautions
Eye/face protection: For tight fitting safety glasses, use equipment tested and approved by official standards such as NIOSH (US) or EN 166 (EU) for eye protection; Skin Protection: Wear Gloves Gloves must be inspected before use; Body Protection: A full set of chemical-resistant work clothes, the type of protective equipment must be selected according to the concentration and quantity of dangerous substances in the specific workplace; Respiratory Protection: If hazard assessments indicate the use of air-purifying respirators, use full-face, multi-purpose respirators (US) or ABEK-type (EN14387) respirators as an alternate to engineering controls. If a gas mask is the only means of protection, use a full-face air supply gas mask. Respirators Use respirators and parts that have been tested and passed government standards such as NIOSH (US) or CEN (EU).
Reference
1 Maciejewska M. Characterization of macroporous N‐vinyl‐2‐pyrrolidone copolymers obtained by suspension polymerization[J]. Journal of applied polymer science, 2012, 124(1): 568-575.
2 Han L M, Timmons R B. Pulsed‐plasma polymerization of N‐vinyl‐2‐pyrrolidone: synthesis of a linear polymer[J]. Journal of Polymer Science Part A: Polymer Chemistry, 1998, 36(17): 3121-3129.
);You may like
Related articles And Qustion
See also
Lastest Price from N-Vinyl-2-pyrrolidone manufacturers

US $40.00-20.00/kg2024-03-11
- CAS:
- 88-12-0
- Min. Order:
- 500kg
- Purity:
- 99.9
- Supply Ability:
- 200tons

US $60.00/kg2024-02-27
- CAS:
- 88-12-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 tons