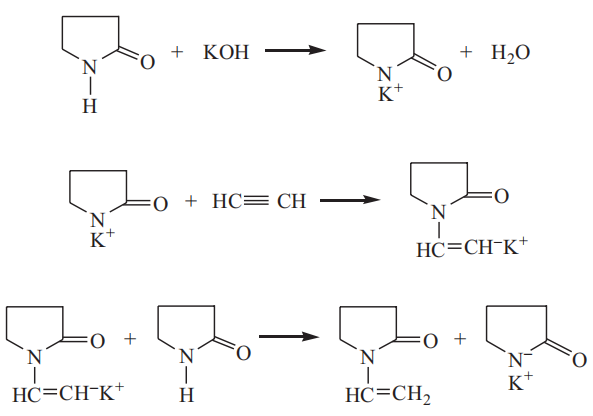
Polymerization and hydrolysis of N-Vinyl-2-pyrrolidone
Description
N-Vinyl-2-pyrrolidone (NVP) is a derivative of 2-pyrrolidone and acetylene. Molecular formula: C6H9NO, molecular weight: 111.1418. It is not difficult to find from the molecular structure of NVP that the NVP molecular structure contains an active unsaturated vinyl group (CH2=CH–), a hydrophilic group (N atom and carbonyl group), and a hydrophobic group (hydrocarbyl group and the cyclic hydrocarbon group). Thus, it has many excellent properties. NVP is produced yearly up to several thousand tons to be used as a monomer and mainly as a starting molecule for producing polyvinyl pyrrolidone, which in turn is widely used in pharmaceutics, cosmetics, and food technology[1].
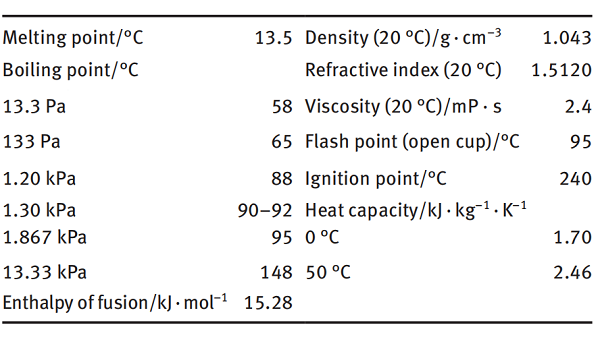
Properties of NVP
NVP is a colorless transparent liquid at room temperature. It is completely miscible with water, alcohol, ether, ester, chlorinated hydrocarbon, and aromatic hydrocarbon, yet it is only partially soluble in aliphatic hydrocarbons. NVP has some special chemical properties because of the vinyl group on its nitrogen atom. The most important of them are easy polymerization and hydrolysis.
Hydrolysis
Under acidic conditions, NVP is easily hydrolyzed to 2-pyrrolidone and acetaldehyde. To prevent NVP products from hydrolysis during production and storage, water must be removed from NVP. The water content in NVP should generally be less than 0.1 wt%. During storage and transportation, a 0.1% base such as sodium hydroxide, ammonia, or a low molecular weight amine can be added to keep the product weakly alkaline for inhibiting hydrolysis or polymerization[2].
Polymerization
The NVP monomer readily undergoes polymerization under the action of heat or an initiator. Even in the absence of an initiator, different degrees of polymerization occur when an NVP sample is left for a long time, which has a negative impact on the quality and reactivity of the monomer. Therefore, a polymerization inhibitor is generally added to the commercially available NVP, and the inhibitor must be removed before the monomer is applied to the polymerization reaction for the production of polyvinylpyrrolidone and other copolymers. The polymerization inhibitor is usually removed from NVP by vacuum distillation or adsorption to obtain pure NVP.
Uses
N-Vinylpyrrolidone is mainly used to synthesize polyvinylpyrrolidone and its copolymers with other monomers. In addition, NVP is used as a low-viscosity reactive diluent monomer in the UV radiation curing process, which can accelerate the UV curing rate. It is usually compatible with acrylates and has a unique viscosity-reducing effect and enhanced adhesion to nonpolar materials. UV-curable coatings containing NVP can impart high abrasion resistance to resin lenses, glass protective films, and credit cards.
References
[1] Franz Oesch. “Enigmatic mechanism of the N-vinylpyrrolidone hepatocarcinogenicity in the rat.” Archives of Toxicology 95 12 (2021): 3717–3744.
[2] Li, Sifang. “Manufacture of Fine Chemicals from Acetylene.” 2021. 0.
);You may like
Related articles And Qustion
See also
Lastest Price from N-Vinyl-2-pyrrolidone manufacturers

US $40.00-20.00/kg2024-03-11
- CAS:
- 88-12-0
- Min. Order:
- 500kg
- Purity:
- 99.9
- Supply Ability:
- 200tons

US $60.00/kg2024-02-27
- CAS:
- 88-12-0
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 100 tons