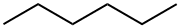Applications of hexane as a non-polar solvent
Description
Hexane is an organic compound with the chemical formula C6H14. It is a natural constituent of crude oil, and in its pure form, n-hexane is a colorless, highly flammable liquid. Hexane is a minor constituent of crude oil and natural gas. Its inclusion in a variety of petroleum products is consequence of refining operations that separate hydrocarbons within specific ranges of boiling points for such uses as heating oils or automotive fuels.
Molecular polarity analysis of Hexane
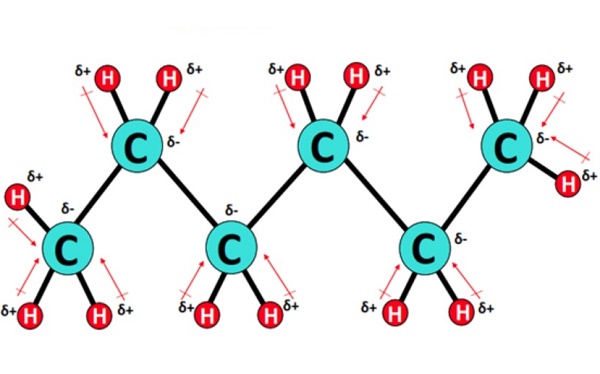
Hexane is non-polar due to being made of non-polar carbon-hydrogen bonds. While there is a slight difference in electronegativity between its hydrogen and carbon atoms, it is not significant enough to be polar. Its symmetrical structure would cancel the polarity even if polar bonds were in the molecule. Precisely, C6H14 consists of 5 C-C single covalent bonds and 13 C-H bonds in an alicyclic or straight-chain arrangement. Each C-C bond is non-polar as zero or no electronegativity difference exists between two identical carbon atoms. Conversely, each C-H bond is very weakly polar, having a slight electronegativity difference of 0.35 units between the bonded atoms. However, due to the symmetrical tetrahedral shape of Hexane w.r.t each C-atom, the small C-H dipole moments get canceled equally on each side of the molecule. Thus, Hexane (C6H14) is overall non-polar (net µ = 0).
Use
In common commercial applications, it is a component of mixed solvents used in various cleaning agents and degreasers or adhesives in the printing, textile, and shoemaking industries. In a highly purified form, n-hexane is used in chemical laboratories as an extractant for a wide range of hydrocarbons and non-polar organic compounds.
Hexane is undoubtedly the most widely used among the solvents used industrially for extracting non-polar edible natural products such as colors, flavors, fragrances, or lipids [1]. N-Hexane is used mainly as an edible oil extractant for a variety of seed crops such as soybeans, cottonseed, rape seed (canola), flax (linseed), mustard seed, peanuts, safflower seed, and corn germ, which are then processed into foods for humans or livestock. While other petroleum-derived solvents (e.g., pentane) or other organic solvents (e.g., chloroform, methanol, ethanol, or ammonia-alcohol mixtures) are currently being studied or are used for certain processes, n-hexane has been widely used since the early part of this century, especially with soybeans, cottonseed, and linseed.
Reference
[1] Christian Cravotto. “Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets.” Foods (2022).
You may like
Related articles And Qustion
See also
Lastest Price from Hexane manufacturers

US $0.00/KG2024-04-28
- CAS:
- 110-54-3
- Min. Order:
- 1KG
- Purity:
- ≥99%
- Supply Ability:
- 2000mt/year

US $0.00/kg2024-04-12
- CAS:
- 110-54-3
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 2000ton