Bromodiphenylmethane-Application
Bromodiphenylmethane is a halogenated building block. Benzhydryl group was preferred to the commoner benzyl to protect 2-nitrophenol in the Bartoli (vinyl Grignard) synthesis of 7-hydroxyindole. Protection was by reaction with the phenol in the presence of potassium carbonate in acetone; deprotection of the indole was by hydrogenolysis[1-3]. Bromodiphenylmethane is a white crystalline solid. A lachrymator. In the presence of moisture corrosive to tissue and most metals. Bromodiphenylmethane is used as a reagent in the synthesis of O-(triazolyl)methyl carbamates as a novel and potent class of fatty acid amide hydrolase (FAAH) inhibitors.Bromodiphenylmethane is a solid with a pungent odor and is tear-prone. Melting point is 47℃. Boiling point is 184℃ (2.67kPa), 150-155℃ (0.267kPa). Bromodiphenylmethane is soluble in alcohol, easily soluble in benzene.
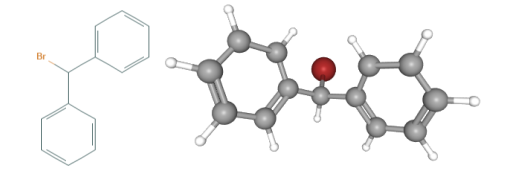
Fig 1. Chemical structure formula and three-dimensional structure of Bromodiphenylmethane
Cinnarizine is made from anhydrous piperazine and brominated diphenylmethane to obtain benzhydryl piperazine, which is then condensed with phenylpropene chloride. Diphenylmethane is heated in the light, bromine is added dropwise, and the temperature is maintained at 130℃ for 1h to obtain bromodiphenylmethane, , melting point 45℃. Bromodiphenylmethane was added dropwise to the piperazine toluene solution, and the mixture was stirred at 80-90℃ for 3 hours. After cooling, the reaction solution was washed with water, and then extracted with 10% dilute hydrochloric acid. The acid layer was alkalized to precipitate out, filtered and dried. Diphenylmethylpiperazine was obtained. It was dissolved in 95% ethanol, sodium carbonate was added, and phenylpropenyl chloride was added dropwise at about 65℃. After the drop was completed, the mixture was heated under reflux for 4 hours, and filtered while hot. After purification, a finished product with a melting point of 117-119℃ is obtained. Based on diphenylmethane, the total yield was 48.2%.
A new type of electrorheological material, namely polymer-type electrorheological fluid using polynaphthoquinone as solid particles and brominated diphenylmethane as liquid.
Propose a mechanism for the Williamson ether synthesis of diphenhydramine hydrochloride (benadryl) from bromodiphenylmethane and 2-(dimethylamino)ethanol. Include all charges and intermediates. Use arrows to clearly show bonds forming or breaking between atoms.The deprotonation of 2-(dimethylamino)ethanol gives alkoxide ion.The so formed alkoxide ion attacks the bromodiphenylmethane to form 2-(benzhydryloxy)-N,N-dimethylethanamine. The reaction follows the bimolecular nucleophilic substitution reaction.The addition of hydrogen chloride to 2-(benzhydryloxy)-N,N-dimethylethanamine gives diphenhydramine hydrochloride (benadryl) as the final product[4].
References
[1]S. Winstein.; Arnold H. Fainberg.; E. Grunwald. Correlation of Solvolysis Rates. VIII. Benzhydryl Chloride and Bromide. Comparison of mY and Swain's Correlations. J. Am. Chem. Soc. 1957, 79 (15), 4146-4155.
[2]Anu Mahadevan.; Howard Sard.; Mario Gonzalez.; John C. McKew. A general method for C3 reductive alkylation of indoles. Tetrahedron Letters. 2003, 44(24), 4589-4591.
[3]The benzhydryl group was preferred to the commoner benzyl to protect 2-nitrophenol in the Bartoli (vinyl Grignard) synthesis of 7-hydroxyindole. Protection was by reaction with the phenol in the presence of potassium carbonate in acetone; deprotection of the indole was by hydrogenolysis: Synth. Commun., 21, 611 (1991).
[4]Paul S, Gupta M. Zinc‐Catalyzed Williamson Ether Synthesis in the Absence of Base.[J]. 2004, 45(48):8825-8829.
);You may like
Related articles And Qustion
See also
Lastest Price from Bromodiphenylmethane manufacturers
US $100.00-1.00/KG2024-03-25
- CAS:
- 776-74-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- g-kg-tons, free sample is available

US $25.00/kg2024-02-27
- CAS:
- 776-74-9
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 5000 tons