Chlorodimethylphenylsilane-Application
Dimethylphenylsilane is a reagent for enol ether synthesis. Dimethylphenylsilane acts as a catalyst. Also used as a precursor in Optical Emission Spectroscopy of Plasma Deposition Processes. Dimethylphenylsilane can react with vinylbenzene to produce (b-Phenyl-ethyl)-dimethylphenyl-silan.
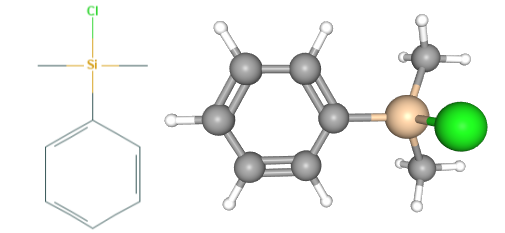
Fig 1. Chemical structure formula and three-dimensional structure of Chlorodimethylphenylsilane
Chlorodimethylphenylsilane is used in the synthesis of enantioenriched allenylsilanes through an ortho-ester Claisen rearrangement of chiral and silylpropargylic alcohols[1,2].
Chlorodimethylphenylsilane may be used in many of the same applications as chlorotrialkylsilanes, but in some cases the presence of the phenyl group on silicon offers advantages. For example, Me2PhSiLi is easier to prepare than Me3SiLi[3].
Chlorodimethylphenylsilane is a useful derivatizing agent for alcohols. DMPSCl derivatized monosaccharides may be analyzed chromatographically with UV detection. A novel application of the UV-absorbing ability of the dimethylphenylsilyloxy chromophore uses 266 nm light absorbed by the DPS `antenna' in 3a-dimethylphenylsilyloxy-5a-androstane-11,17-dione for photolytic reduction of the C-17 keto group. Photolysis with 308 nm light absorbed by the ketone moieties produces predominately D-ring epimerization[4-6].
Chlorodimethylphenylsilane may be used to trap ester enolates (however, lactam enolates silylate at carbon). Numerous C-silylations of organolithium compounds with Dimethylphenylsilane have been reported. Other compounds derived from Dimethylphenylsilaneinclude the azide, tributylstannane, cyanide, isocyanate, and isoselenocyanate.
Another advantage of the dimethylphenylsilyl group over trialkylsilyl is that the phenyl substituent may be readily removed by protodesilation. Kumada-Fleming-Tamao oxidation converts C-silyl compounds into alcohols. Allylic dimethylphenylsilanes are preferred substrates for conversion to the corresponding allylic alcohols, which is the basis for use of the b-silylethyl vinyl ketone as a b-hydroxyethyl vinyl ketone synthon in the Robinson annulation reaction[7,8].
References
[1]Boukachabia, M.; Vriamont, N.; Lambin, D.; Riant, O.; Aribi-Zouioueche, L. Synthesis and reactions of donor cyclopropanes: efficient routes to cis- and trans-tetrahydrofurans. Tetrahedron 2015, 71 (39), 7386-7414.
[2]Dunn, J.; Dobbs, A. P. Chlorobenzene Poisoning and Recovery of Platinum-Based Cathodes in Proton Exchange Membrane Fuel Cells. J. Phys. Chem. C 2015, 119 (35), 20328-20338.
[3]Ian Fleming, Rolf Henning, Howard Plaut. The phenyldimethylsilyl group as a masked form of the hydroxy group[J]. Journal of the Chemical Society Chemical Communications, 1984, 26(1):29-31.
[4]Harvey D J. Comparison of fourteen substituted silyl derivatives for the characterization of alcohols, steroids and cannobinoids by combined gas—liquid chromatography and mass spectrometry[J]. 1978, 147(JAN):291-298.
[5]Zhu, H, Zhu, G, Liu, J. Rabaptin-5-independent Membrane Targeting and Rab5 Activation by Rabex-5 in the Cell[J]. Molecular Biology of the Cell, 18(10):4119-4128.
[6]White C A, Vass S W. Analysis of phenyldimethylsilyl derivatives of monosaccharides and their role in high-performance liquid chromatography of carbohydrates[J]. 1983, 264(1):99-109.
[7] Chuy, N. D.; Chvalovsky, V.; Schraml, J.; Magi, M.; Lippmaa, E. CCC 1975, 40, 875.
[8]Morizawa, Y.; Oda, H.; Oshima, K.; Nozaki, H. TL 1984, 25, 1163.
);You may like
See also
Lastest Price from Chlorodimethylphenylsilane manufacturers

US $0.00-0.00/KG2023-01-10
- CAS:
- 768-33-2
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 20 mt
US $0.00-0.00/KG2022-10-01
- CAS:
- 768-33-2
- Min. Order:
- 1KG
- Purity:
- 98%
- Supply Ability:
- 1ton
![3528-45-8 Application; 1-[(4-Methoxyphenyl) methyl]-1H-pyrazol-5-amine; 2-(4-Methoxy-benzyl)-2H-pyrazol-3-ylam](httpss://www.chemicalbook.com/NewsImg/2019-12-27/201912271427157737.jpg)
