Homoprotocatechuic acid - Application, Synthesis and Safety
Homoprotocatechuic acid, (3,4-dihydroxyphenyl)acetic acid is a dihydroxyphenylacetic acid having the two hydroxy substituents located at the 3- and 4-positions. It is a metabolite of dopamine. It has a role as a human metabolite. It is a dihydroxyphenylacetic acid and a member of catechols. It derives from a phenylacetic acid. It is a conjugate acid of a (3,4-dihydroxyphenyl)acetate. 3,4-dihydroxyphenylacetic acid, commonly known as DOPAC, one of the most important dopamine metabolites. Despite the role it plays in various molecular mechanisms of biochemical importance.
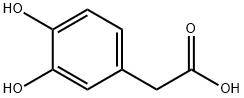
The molecular structure of DOPAC is depicted in Fig. 1. It is constituted by an aromatic ring bearing an acetic acid side chain on one side and two vicinal hydroxyl groups on the other side. Hence, DOPAC can be considered as a catechol (ortho-dihydroxyphenyl) ring with an acid side chain or, alternatively, as a phenylacetic acid augmented with a dihydroxy moiety. It appears interesting to investigate how the molecular properties of DOPAC depend on its two side chains (acetic and dihydroxy) and if they can be considered additive or cooperative.[1]
The conformational characterization of 3,4-dihydroxyphenylacetic acid (DOPAC) has been undertaken by B3LYP and MP2 theoretical methods, and matrix-isolation infrared spectroscopy. The theoretical calculations allowed identifying thirteen unique DOPAC conformers, the five lowest energy ones being predicted to account for up 99.7% of the gas phase conformational equilibrium at 298.15 K.[3]


Figure 1 the molecular formula of Homoprotocatechuic acid
Application
3, 4-Dihydroxyphenylacetic acid (3, 4-DHPA) is a phenolic acid with strong anti-oxidative activity, showing potential applications in food and pharmaceutical industries. Phenolic acids are widely used in food, cosmetic, and pharmaceutical industries because of their anti-oxidative, antibacterial and other biological activities 3, 4-Dihydroxyphenylacetic acid (3, 4-DHPA) is a naturally-occurring phenolic acid exhibiting strong free radical scavenging activity. It can reduce lipid peroxidation and may be potentially used as a natural antioxidant to replace butylated hydroxytoluene (BHT) and butylated hydroxyanisol (BHA) for stabilization of edible oils. 3, 4-DHPA also shows several health-benefit activities. It is a predominant biologically-active catabolite of dietary quercetin glycosides and exhibits protective effects against apoptosis, mitochondrial dysfunction and oxidative stress.
In addition, it is shown that 3, 4-DHPA can inhibit the secretion of the pro-inflammatory cytokines in lipopolysaccharide-stimulated peripheral blood mononuclear cells and the expression of P-selectin in resting platelets. 3,4-Dihydroxyphenylacetic acid is one of the major colonic microflora-produced catabolites of quercetin glycosides, such as quercetin 4′-glucoside, rutin, and hyperoside (quercetin 3-galactoside). Human fecal bacteria have the ability to catalyze the formation of DOPAC,and an excretion of DOPAC was increased in human urine after the digestion of polyphe-nols from chocolate, suggesting the actual occurrence of DOPAC in humans. We have recently identified DOPAC as a predominant antioxidative catabolite of quercetin glycosides. DOPAC also inhibited the hydrogen peroxide-induced cytotoxicity in hepatocytes. In addition to its antioxidant-related activities, DOPAC inhibited the secretion of pro-inflammatory cytokines from peripheral blood mononuclear cells.
Synthesis
A 3, 4-DHPA biosynthetic pathway was designed by connecting 4-hydroxyphenylacetic acid (4-HPA) biosynthesis with its hydroxylation. 3, 4-DHPA production by electrochemical conversion of phenylacetic acid (PA) was investigated, leading to the formation of a mixture of dihydroxylated PAs. Fortunately, enzymatic hydroxylation can be easily achieved with good specificity. Tyrosol and 4-hydroxyphenylacetic acid (4-HPA) are analogues and synthetic precursors of 3, 4-DHPA. Halomonas sp. strain HTB24 was used as the whole-cell biocatalyst, which produced 5.15 mM 3, 4-DHPA from 20 mM tyroso. Immobilized mushroom tyrosinase was used to convert 4-HPA to 3, 4-DHPA. However, the enzyme preparation is not cost effective and
excess ascorbic acid is required to prevent its over-oxidation to form oquinones. By introducing natural pathways into genetically tractable micro-organisms or even designing artificial pathways, efficient production of target compounds can be achieved from simple and renewable carbon sources . Over the years, an increasing number of phenolic compounds such as caffeic acid, salvianic acid, gallic acid and pyrogallol have been successfully produced via metabolic engineering approaches. However, de novo biosynthesis of 3, 4-DHPA has not been reported before. 4-HPA is a synthetic precursor of 3, 4-DHPA. A pathway was designed for 4-HPA biosynthesis. In this pathway, E. coli endogenous metabolite 4-hydro-xyphenylpyruvate (4-HPP) is converted to 4-HPA by two sequential. It designed a novel biosynthetic pathway and achieved 3, 4-DHPA production from simple carbon sources for the first time. In addition, the production efficiency was greatly improved by enhancing the upstream pathway and disrupting pykA and pykF. This work provides a promising method for sustainable production of 3, 4-DHPA.[2]
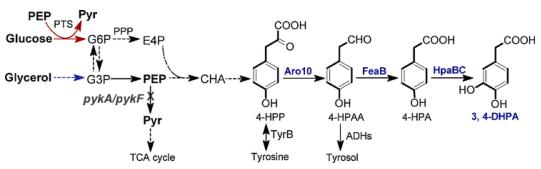
Figure2. Biosynthetic pathway of 3, 4-dihydrox-yphenylacetic acid
Toxicological and Safety
Homoprotocatechuic acid modulates the aldehyde dehydrogenase (ALDH) activity and protects the cells from the acetaldehyde-induced cytotoxicity in vitro. Homoprotocatechuic acid was shown to enhance not only the total ALDH activity, but also the gene expression of ALDH1A1, ALDH2 and ALDH3A1 in a concentration-dependent manner. Homoprotocatechuic acid simultaneously stimulated the nuclear translocation of NFE2-related factor 2 and aryl hydrocarbon receptor. The pretreatment of Homoprotocatechuic acid completely protected the cells from the acetaldehyde-induced cytotoxicity. The present study suggested that Homoprotocatechuic acid acts as a potential ALDH inducer to prevent the alcohol-induced abnormal reaction.
the possibility of Homoprotocatechuic acid as a potential enhancer of the ALDH activity, we examined the protective effect of Homoprotocatechuic acid on the acetaldehyde-induced cytotoxicity in vitro. The results showed that Homoprotocatechuic acid increased the total ALDH activity as well as nuclear protein levels of the transcriptional factor NFE2-related factor 2 (Nrf2) and aryl hydrocarbon receptor (AhR). Furthermore, the pretreatment of Homoprotocatechuic acid increased the resistance to the acetaldehyde-induced cytotoxicity. It identified a major catabolite of quercetin glycosides, Homoprotocatechuic acid, as an ALDH activity enhancer. The Homoprotocatechuic acid pretreatment also increases the resistance to the acetaldehyde-induced cytotoxicity, possibly through the transcriptional regulation of the ALDH genes by Nrf2 and AhR. Since Homoprotocatechuic acid is a phenolic acid catabolite of dopamine as well as quercetin with a much lower cytotoxicity, Homoprotocatechuic acid has some advantages for application as a food chemical to prevent humans from an alcohol-induced abnormal reaction. Future efforts will be concerned with further understanding the signaling pathway of the ALDH induction as well as in vivo significance of the protective effect of Homoprotocatechuic acid against the alcohol-induced toxicity.[3]
References
1.Liu Y., Kurita A. & Nakashima S. et al., "3,4-Dihydroxyphenylacetic acid is a potential aldehyde dehydrogenase inducer in murine hepatoma Hepa1c1c7 cells," Biosci Biotechnol Biochem, Vol.81, No.10(2017), pp.1978-1983.
2.Li X., Shen X. & Wang J. et al., "Efficient biosynthesis of 3, 4-dihydroxyphenylacetic acid inEscherichia coli," Journal of Biotechnology, Vol.294(2019), pp.14-18.
3
);You may like
See also
Lastest Price from 3,4-Dihydroxyphenylacetic acid manufacturers

US $0.00/kg2023-12-11
- CAS:
- 102-32-9
- Min. Order:
- 1kg
- Purity:
- 0.99
- Supply Ability:
- 20 tons

US $0.00/KG2023-11-16
- CAS:
- 102-32-9
- Min. Order:
- 1KG
- Purity:
- 99%
- Supply Ability:
- 50000KG/month