Methylaminoformyl chloride-Application
Methylaminoformyl chloride(C2H4ClNO) is used as a reagent in the synthesis of acylprolinamides as a new class of peptide deformylase inhibitors with in vivo antibacterial activity. Methylaminoformyl chloride is also used as a reagent in the synthesis of carbamate derivatives as potential dual-binding site acetylcholinesterase inhibitors. The decomposition tail gas hydrogen chloride of Methylaminoformyl chloride is also useful during the cartap synthesis[1].
Methylaminoformyl chloride is white crystal, m.p. 45°C (decomposition), easily soluble in organic solvents such as benzene. Methylaminoformyl chloride is easily decomposed by alkali when Methylaminoformyl chloride is decomposed by water. Methylaminoformyl chloride is unstable under high temperature and is irritating to eyes and respiratory tract.
Methylaminoformyl chloride is liquid at 35°C or lower, and is gaseous at 40°C or higher, and
Methylaminoformyl chloride is easily soluble in carbon tetrachloride, chlorobenzene, and the like.
Methylaminoformyl chloride is produced by reacting methylamine with phosgene. Methylaminoformyl chloride is an intermediate of carbamate pesticides and can be used to produce insecticides such as carbaryl.
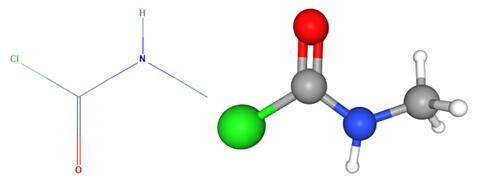
Fig 1. Chemical structure formula and three-dimensional structure of Methylaminoformyl chloride
Methylaminoformyl chloride is prepared by reacting methylamine with phosgene. Reaction equation: CH3NH2+COCl2→CH3NHCOCl. A 40% aqueous solution of methylamine is vaporized, and after drying, it is combined with phosgene at a ratio of 1:1.3 (methanol), methylamine at 4 m3/h, and phosgene at a rate of 8.6 m3/h (content 60%-70%). Preheating into the preheater, the preheating temperature of methylamine is controlled at 220-260°C, and the phosgene is controlled at 200-240°C. The preheated two gases enter the test tube and are synthesized at 280-300°C to obtain gaseous methaqualyl chloride. Then, carbon tetrachloride (or chlorobenzene solution) is circulated and absorbed at 0-20°C to obtain a solution of about 10% of carbamoyl chloride (or chlorobenzene), or cooled to a liquid product after 35-40°C or lower[2,3].
Methylaminoformyl chloride can be widely used as an intermediate of carbamate insecticides such as Zhongdingwei, carbofuran, isoprocarb, methomyl, chlorhexidine and carbaryl.
References
[1] Hu Xiao, et al. "Application method of methylamino formyl chloride decomposition tail gas hydrogen chloride in cartap synthesis.", CN 103848768 A. 2014.
[2] Verma A. Small Core Heterocyclic Carbamates and Carboxamides: Resistance-breaking Acetylcholinesterase Inhibitors Targeting the Malaria Mosquito, Anopheles gambiae[J]. 2014.
[3] Beierl D , Schmidt A . Zur Reaktion von N‐Methyl‐ und N,N‐Dimethylcarbamidsäurechlorid mit Antimon(V)‐chlorid[J]. Chemische Berichte, 1973, 106(5):1637-1642.
You may like
See also
Lastest Price from Methylaminoformyl chloride manufacturers

US $10.50/kg2024-02-27
- CAS:
- 6452-47-7
- Min. Order:
- 1kg
- Purity:
- 90%
- Supply Ability:
- 500TM/month