Polarity of Acetaminophen
Acetaminophen Hydrophilic
Acetaminophen is a poorly water-soluble drug whose solubility in water can be increased by adding cosolvents.The study reported the effect of three natural deep eutectic solvents (NADESs), choline chloride + L-malic acid, choline chloride + malonic acid and choline chloride + urea, on the aqueous solubility of acetaminophen in the temperature range of 293.15 K to 323.15 K. The study was carried out in the presence of three natural deep eutectic solvents (NADESs).
The results showed that the solubility of acetaminophen increased with increasing temperature or with increasing concentration of the studied co-solvents. The most and least effective co-solvents were choline chloride and urea, respectively. For example, moles of 6.45% choline chloride in aqueous solution increased the solubility of acetaminophen to more than four times the solubility in deionised water. The solubility of acetaminophen in aqueous solutions of the components of NADESs was used to estimate the NRTL binary interaction parameters for these systems. The model with an AARD of 3.2% was used to calculate the solubility of acetaminophen in aqueous solutions of each component of NADESs.
The binary interaction parameters were then used to predict the solubility of acetaminophen in aqueous solutions of NADESs. The model predicted the solubility of acetaminophen in aqueous solutions of NADESs with an AARD of 6.2%, which is reliably predictable.
Acetaminophen Separated by column chromatography
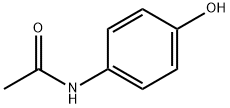
Column chromatography can be used to separate the components acetaminophen, aspirin and caffeine. When using a polar immobile phase and a less polar mobile phase, the elution of the compounds occurs based on the relative polarities of the components. Based on the polarities of the components above, aspirin, being the least polar, will elute first; acetaminophen will follow, and caffeine will shortly after acetaminophen.
Acetaminophen has a phenol and amide function group, but caffeine has multiple amide functional groups; therefore, acetaminophen is more polar than aspirin and less polar than caffeine. As a consequence, acetaminophen will elute second, and caffeine will elute last.
References:
[1] SARA HAJEBRAHIMI Aliakbar R. Solubility of acetaminophen in aqueous solutions of three natural deep eutectic solvents (NADESs) and individual components of the NADESs[J]. Journal of Molecular Liquids, 2020, 316: Article 113867. DOI:10.1016/j.molliq.2020.113867.
);You may like
Related articles And Qustion
Lastest Price from Acetaminophen manufacturers

US $1.00/kg2024-04-27
- CAS:
- 103-90-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10 tons

US $10.00/kg2024-04-26
- CAS:
- 103-90-2
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 10000 tons