Pyridine: Uses & Synthesis
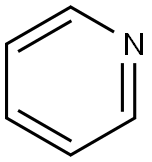
Pyridine (molecular formula C6H5N) contains a six-membered heterocyclic compound with a nitrogen heteroatom, that is, a compound formed by replacing one -CH= with nitrogen in the benzene molecule. Similar to benzene, it has the same electronic structure and is still aromatic, so Also known as azabenzene and azabenzene, it is a colorless liquid with special odor at room temperature. It has a melting point of -41.6°C and a boiling point of 115.2°C. It forms an azeotropic mixture with water and has a boiling point of 92-93°C. (Industrial use of this property to purify pyridine), density 0.9819g/cm3. Easily soluble in most organic solvents such as water, ethanol, ether, etc., it can also be used as a solvent by itself. Pyridine was first separated from bone tar, and later it was discovered that coal tar, coal gas, shale oil, and petroleum also contained pyridine and its homologs, such as 2-methylpyridine and 2,6-lutidine.
Uses
Pyridine can also be used as a denaturant, dye assistant, and starting material for the synthesis of a series of products, including medicines, disinfectants, dyes, food seasonings, adhesives, explosives and so on. Pyridine is toxic. Inhalation, ingestion, or skin contact can reduce male fertility and can also cause cancer. Pyridine is a weak tertiary amine, which can form water-insoluble salts with various acids (such as picric acid or perchloric acid) in ethanol solution. The pyridine used in industry contains about 1% 2-methylpyridine, so the difference in salt-forming properties can be used to separate it from its homologues. Pyridine can also form crystalline complexes with a variety of metal ions.
Pyridine is easier to reduce than benzene. For example, it can be reduced to hexahydropyridine (or piperidine) under the action of sodium metal and ethanol. Pyridine reacts with hydrogen peroxide and is easily oxidized to N-oxide pyridine. N-oxide pyridine is an important pyridine derivative. After the nitrogen atom is oxidized, it can no longer form a positively charged pyridine ion, which is beneficial to the generation of aromatics. In the family electrophilic substitution reaction, after the substitution is completed, the oxygen on the nitrogen is removed to obtain derivatives that cannot be obtained by direct substitution of pyridine.
Synthesis
Pyridine was previously extracted from coal tar. The coke oven gas was washed with sulfuric acid and neutralized with ammonia to obtain crude light pyridine, which was then purified by distillation. With the expansion of the use of pyridine, the synthetic method of pyridine has developed greatly. Pyridine and pyridyl compounds obtained from coal tar abroad account for 10%. A mixture of acetaldehyde (1.648mol), 37% formaldehyde (1.665mol) and ammonia (3.096mol) was reacted at 432°C. The catalyst was SiO2-Al2O3-Bi2O3, and the pyridine yield was 48.4%. At the same time, β-picoline is also generated, and the yield of pyridine, picoline and picoline can be adjusted by changing the operating conditions. In addition, 1,5-pentanediamine hydrochloride can be cyclized by heating and dehydrogenated in the presence of a Pt catalyst to produce pyridine
);You may like
Related articles And Qustion
See also
Lastest Price from Pyridine manufacturers

US $30.00-22.00/kg2024-04-26
- CAS:
- 110-86-1
- Min. Order:
- 1kg
- Purity:
- 99%
- Supply Ability:
- 20Tons
US $1.10/g2024-04-17
- CAS:
- 110-86-1
- Min. Order:
- 1g
- Purity:
- 99.0% min
- Supply Ability:
- 100 tons min