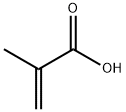
Synthesis of Methacrylic acid
General description
Methacrylic acid appears as a clear colorless liquid (or low-melting solid) with a pungent odor. Corrosive to metals and tissue. Less dense than water and is soluble in water. Vapors heavier than air. Used to make plastics. Methacrylic acid is an alpha,beta-unsaturated monocarboxylic acid that is acrylic acid in which the hydrogen at position 2 is substituted by a methyl group. It derives from an acrylic acid and a conjugate acid of a methacrylate.[1]The thermal stability, char yield and flame retardancy have been obviously improved by grafting acrylic or methacrylic acid or their sodium salts into ABS or SBS.
Application
Methyl methacrylate (MMA) is a specialty monomer for poly methyl methacrylate (PMMA) and the increasing demand for this monomer has motivated industry to develop clean technologies based on renewable resources. The dominant commercial process reacts acetone and hydrogen cyanide to MMA (ACH route) but the intermediates (hydrogen cyanide, and acetone cyanohydrin) are toxic and represent an environmental hazard.
Synthesis
1.A catalyst for directly preparing methacrylic acid by one-step method using isobutylene as a raw material and a preparation method thereof. The catalyst contains two different active components, Mo-Bi metal oxide and mesoporous Mo-P heteropolyacid, which can selectively oxidize isobutene to methacrolein and further oxidize methacrolein to methacrylic acid, respectively. The preparation method of the catalyst is divided into two steps. After the Mo-Bi metal oxide particles are prepared, they are dispersed in a Mo-P heteropolyacid precursor solution containing a surfactant and a silica precursor again to obtain a composite catalyst. The heat treatment method of the catalyst is also divided into two steps, namely, calcination in an inert gas and an oxidizing gas atmosphere respectively, and finally a catalyst with good thermal stability is obtained. The catalyst can realize the direct oxidation of isobutene to methacrylic acid in one step, and has good reactivity and thermal stability[2].
2. Catalysis for the synthesis of methacrylic acid:Esterification of methacrylic acid (MAA) to MMA is a compelling alternative together with ethylene, propylene, and isobutene/t-butanol as feedstocks. Partially oxidizing isobutane or 2-methyl-1,3-propanediol (2MPDO) over heteropolycompounds to MAA in a single-step is nascent technology to replace current processes. The focus of this review is on catalysts and their role in the development of processes herein described. Indeed, in some cases remarkable catalysts were studied that enabled considerable steps forward in both the advancement of catalysis science and establishing the basis for new technologies. An emblematic example is represented by Keggin-type heteropolycompounds with cesium and vanadium, which are promising catalysts to convert isobutane and 2MPDO to MAA. Renewable sources for the MMA or MAA route include acetone, isobutanol, ethanol, lactic, itaconic, and citric acids. End-of-life PMMA is expected to grow as a future source of MMA[3].

Figure 1 Common ACH approach to produce Methyl methacrylate
Safety
Route of entry: inhalation, ingestion, percutaneous absorption. Health hazards: This product is irritating to the nose and throat; exposure to high concentrations may cause changes in the lungs. Irritant to skin, can cause burns. Eye contact can cause burns and permanent damage. Chronic Effects: May cause lung, liver and kidney damage. It is sensitizing to the skin. After sensitization, even exposure to very low levels of this product can cause skin itching and rashes. Toxicological data and environmental behavior Acute toxicity: LD501600mg/kg (rat oral); 500mg/kg (rabbit percutaneous) Subacute and chronic toxicity: rat inhalation 4.5g/m3, 5 hours, 5 times, nose and eyes appear Irritation, weight loss, blood and urine tests were normal, and visceral dissection was normal. Mutagenicity: DNA damage: Escherichia coli 50umol/L.Hazardous characteristics: In case of fire and high heat, it will cause combustion and explosion. It can react with oxidants. In case of high heat, a polymerization reaction may occur, resulting in a large amount of exothermic phenomenon, causing container rupture and explosion accidents.
Reference
1.Lebeau J., Efromson J. P. & Lynch M. D., "A Review of the Biotechnological Production of Methacrylic Acid," Front Bioeng Biotechnol, Vol.8(2020), p.207.
2.A composite catalyst for the manufacture of methacrylic acid and its preparation method_Wanli.
3.Darabi M. M., Dubois J. L. & Cavani F. et al., "Catalysis for the synthesis of methacrylic acid and methyl methacrylate," Chem Soc Rev, Vol.47, No.20(2018), pp.7703-7738.
);You may like
See also
Lastest Price from Methacrylic acid manufacturers

US $1.00/g2024-04-25
- CAS:
- 79-41-4
- Min. Order:
- 1g
- Purity:
- 99
- Supply Ability:
- 20tons

US $20.00-15.00/L2024-01-08
- CAS:
- 79-41-4
- Min. Order:
- 1L
- Purity:
- 99.60%
- Supply Ability:
- 50tons