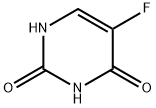
The pharmaceutical analysis of 5-fluorouracil
Introduction
5-Fluorouracil (5-FU) is widely used in the treatment of cancer[1]. Over the past 20 years, increased understanding of the mechanism of action of 5-FU has led to the development of strategies that increase its anticancer activity. Despite these advances, drug resistance remains a significant limitation to the clinical use of 5-FU. Emerging technologies, such as DNA microarray profiling, have the potential to identify novel genes that are involved in mediating resistance to 5-FU. Such target genes might prove to be therapeutically valuable as new targets for chemotherapy, or as predictive biomarkers of response to 5-FU-based chemotherapy.

Picture 1 5-Fluorouracil powders
Development
5-Fluorouracil, first introduced as a rationally synthesised anticancer agent 30 years ago, continues to be widely used in the management of several common malignancies including cancer of the colon, breast and skin. This drug, an analogue of the naturally occurring pyrimidine uracil, is metabolised via the same metabolic pathways as uracil. Although several potential sites of antitumour activity have been identified, the precise mechanism of action and the extent to which each of these sites contributes to tumour or host cell toxicity remains unclear[2].
Application
Several assay methods are available to quantify 5-fluorouracil in serum, plasma and other biological fluids. Unfortunately, there is no evidence that plasma drug concentrations can predict antitumour effect or host cell toxicity[3]. The recent development of clinically useful pharmacodynamic assays provides an attractive alternative to plasma drug concentrations, since these assays allow the detection of active metabolites of 5-fluorouracil in biopsied tumour or normal tissue.
5-Fluorouracil is poorly absorbed after oral administration, with erratic bioavailability. The parenteral preparation is the major dosage form, used intravenously (bolus or continuous infusion). Recently, studies have demonstrated the pharmacokinetic rationale and clinical feasibility of hepatic arterial infusion and intraperitoneal administration of 5-fluorouracil. In addition, 5-fluorouracil continues to be used in topical preparations for the treatment of malignant skin cancers.
Drug
Following parenteral administration of 5-fluorouracil, there is rapid distribution of the drug and rapid elimination with an apparent terminal half-life of approximately 8 to 20 minutes. The rapid elimination is primarily due to swift catabolism of 5-fluorouracil in the liver.
As with all drugs, caution should be used in administering 5-fluorouracil in various pathophysiological states. In general, however, there are no set recommendations for dose adjustment in the presence of renal or hepatic dysfunction. Drug interactions continue to be described with other antineoplastic drugs, as well as with other classes of agents.
The syndrome of 5-fluorouracil cardiotoxicity
The drug 5-fluorouracil (5-FU) is an antimetabolite active against many solid tumors. Most physicians are familiar with its gastrointestinal and bone marrow toxicity[4]. Cardiotoxicity, well recognized for other antineoplastic agents, has been reported after 5-FU therapy with increasing frequency, and the incidence is probably higher.
The elusive aspect of the cardiotoxicity of 5-FU and doxorubicin lies in the typical clinical picture of angina and electrocardiographic changes simulating an ischemic event, whereas significant coronary disease or vasospasm often is not substantiated, and cardiac enzyme levels can remain normal. Because more common cardiomyopathies are not usually painful, we believe that 5-FU-induced angina may have a final pathway in common with ischemic heart disease. Another interesting correlation is the finding that an entity like muscle phosphorylase deficiency can produce intermittent claudication.
Frequent assessment of cardiac function seems prudent for those who receive high-dose 5-FU therapy. This group probably would benefit from continuous electrocardiographic monitoring and intermittent assessment of the ejection fraction. If unexplained angina, static or dynamic electrocardiographic changes, hypo[1]tension, or congestive heart failure develop, a temporary or complete discontinuation of treatment should be considered. In the absence of other suspected cardiotoxins, a rechallenge with 5-FU should be reserved for those patients in whom there is no reasonable alternative treatment, while applying aggressive prophylaxis with nitrates and or calcium-channel blockers. The syn[1]drome is usually reversible and responds favorably to conventional therapy for angina and congestive heart failure after discontinuation of the 5-FU.
Reference
1 Longley D B, Harkin D P, Johnston P G. 5-fluorouracil: mechanisms of action and clinical strategies[J]. Nature reviews cancer, 2003, 3(5): 330-338.
2 Diasio R B, Harris B E. Clinical pharmacology of 5-fluorouracil[J]. Clinical pharmacokinetics, 1989, 16(4): 215-237.
3 Parker W B, Cheng Y C. Metabolism and mechanism of action of 5-fluorouracil[J]. Pharmacology & therapeutics, 1990, 48(3): 381-395.
4 Robben N C, Pippas A W, Moore J O. The syndrome of 5‐fluorouracil cardiotoxicity. An elusive cardiopathy[J]. Cancer, 1993, 71(2): 493-509.
);You may like
Related articles And Qustion
Lastest Price from 5-Fluorouracil manufacturers

US $100.00/KG2024-04-28
- CAS:
- 51-21-8
- Min. Order:
- 25KG
- Purity:
- 99%
- Supply Ability:
- 20tons

US $9.00-70.00/g2024-04-28
- CAS:
- 51-21-8
- Min. Order:
- 10g
- Purity:
- 99%
- Supply Ability:
- 10 tons