General Description
A clear colorless aqueous solution.
Reactivity Profile
SODIUM CYANIDE SOLUTION(143-33-9) is weakly basic. Reacts with acids of all kinds to generate quantities of very poisonous hydrogen cyanide gas. Incompatible with strong oxidizing agents, especially if solution dries out. Gives insoluble products with silver(I), mercury(I) and lead(II) ions that may decompose violently under certain conditions.
Air & Water Reactions
Slowly evolves flammable and poisonous hydrogen cyanide gas.
Hazard
Toxic by ingestion and inhalation.
Health Hazard
Sodium cyanide is a white crystalline solid that is odorless when dry, but emits a slight
odor of hydrogen cyanide in damp air. It is slightly soluble in ethanol and formamide. It is very poisonous. It explodes if melted with nitrite or chlorate at about 450°F. It produces
a violent reaction with magnesium, nitrites, nitrates, and nitric acid. On contact with acid,
acid fumes, water, or steam, it produces toxic and flammable vapors. Synonyms for sodium
cyanide are hydrocyanic acid, sodium salt, and cyanide of sodium.
Potential Exposure
Sodium cyanide is used as a solid or in solution to extract metal ores, in electroplating and metal cleaning baths; in metal hardening; in treatment of rabbit and rat burrows and holes and termite nests; in insecticides
First aid
If this chemical gets into the eyes, remove any contact lenses at once and irrigate immediately for at least 15 minutes, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts the skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove from exposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR if heart action has stopped. Transfer promptly to a medical facility. When this chemical has been swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit. Use amyl nitrate capsules if symptoms develop. All area employees should be trained regularly in emergency measures for cyanide poisoning and in CPR. A cyanide antidote kit should be kept in the immediate work area and must be rapidly available. Kit ingredients should be replaced every 1�2 years to ensure freshness. Persons trained in the use of this kit; oxygen use, and CPR must be quickly available.
Shipping
UN1689 Sodium cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Incompatibilities
Sodium cyanide decomposes on contact with acids, acid salts, water, moisture, alcohols, and carbon dioxide, releasing highly toxic and flammable hydrogen cyanide gas. Aqueous solution is a strong base; it reacts violently with acid and is corrosive. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides. Absorbs moisture from the air forming a corrosive syrup. Corrosive to active metals, such as aluminum, copper, and zinc. Under acid conditions, sarin hydrolyzes to form hydrofluoric acid.
Description
Sodium cyanide, NaCN, is a cyanide salt that is a white, deliquescent, crystalline powder and is soluble in water. The specific gravity is 1.6, which is heavier than water. Sodium cyanide is toxic by inhalation and ingestion, with a TLV of 4.7 ppm and 5 mg/m3 of air. The target organs are the cardiovascular system, central nervous system, kidneys, liver, and skin. Reactions with acids can release flammable and toxic hydrogen cyanide gas. Cyanides are incompatible with all acids. The four-digit UN identification number is 1689.
The NFPA 704 designation is health 3, flammability 0, and reactivity 0. The primary uses are in gold and silver extraction from ores, electroplating, fumigation, and insecticides.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform to EPA regulations governing storage, transportation, treatment, and waste disposal. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office. Add strong alkaline hypochlorite and react for 24 hours. Then flush to sewer with large volumes of water.
Physical properties
Physical Properties White cubic crystals; hygroscopic; density 1.6 g/cm3; melts at 563°C; very soluble in water; aqueous solution strongly alkaline and decomposes rapidly.
Definition
sodium cyanide: A white orcolourless crystalline solid, NaCN,deliquescent, soluble in water and inliquid ammonia, and slightly solublein ethanol; cubic; m.p. 564°C; b.p.1496°C. Sodium cyanide is now madeby absorbing hydrogen cyanide insodium hydroxide or sodium carbonatesolution. The compound is extremelypoisonous because it reacts with the iron in haemoglobin in theblood, so preventing oxygen reachingthe tissues of the body. It is used inthe extraction of precious metals andin electroplating industries. Aqueoussolutions are alkaline due to salt hydrolysis.
Production Methods
Sodium cyanide was first prepared in 1834 by heating
Prussian Blue, a mixture of cyanogen compounds of iron,
and sodium carbonate and extracting sodium cyanide from
the cooled mixture using alcohol. Sodium cyanide remained
a laboratory curiosity until 1887, when a process was patented
for the extraction of gold and silver ores by means of a dilute solution of cyanide.
Reactions
Sodium cyanide, NaCN, white solid, soluble, very poisonous, formed (1) by reaction of sodamide and carbon at high temperature, (2) by reaction of calcium cyanamide and sodium chloride at high temperature, reacts in dilute solution in air with gold or silver to form soluble sodium gold or silver cyanide, and used for this purpose in the cyanide process for recovery of gold. The percentage of available cyanide is greater than in potassium cyanide previously used. Used as a source of cyanide, and for hydrocyanic acid.
Flammability and Explosibility
Sodium cyanide and potassium cyanide are noncombustible solids. Reaction with
acids liberates flammable HCN.
Industrial uses
sodium
cyanide and other water-soluble cyanides are used as modifying reagents for selective
flotation of ores containing galena, sphalerite and gangue minerals.
storage
In
particular, work with cyanides should be conducted in a fume hood to prevent
exposure by inhalation, and splash goggles and impermeable gloves should be worn
at all times to prevent eye and skin contact. Cyanide salts should be stored in a cool,
dry location, separated from acids.
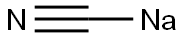
 ;
;
