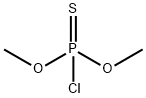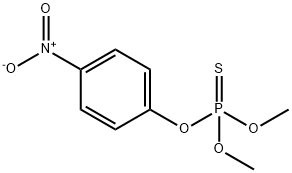
Parathion-methyl
- Product NameParathion-methyl
- CAS298-00-0
- CBNumberCB3348228
- MFC8H10NO5PS
- MW263.21
- EINECS206-050-1
- MDL NumberMFCD00041813
- MOL File298-00-0.mol
Chemical Properties
| Melting point | 36°C |
| Boiling point | 143°C (1.0 mmHg) |
| Density | 1.36 |
| vapor pressure | 4.1×10-4 Pa (20 °C) |
| refractive index | nD25 1.5367 |
| Flash point | 46.1°C |
| storage temp. | APPROX 4°C |
| solubility | Chloroform: Slightly Soluble,Methanol: Slightly Soluble |
| form | solid |
| Water Solubility | 0.005 g/100 mL |
| BRN | 8905814 |
| Stability | Stable. Flammable. |
| CAS DataBase Reference | 298-00-0(CAS DataBase Reference) |
| FDA UNII | 41BCL2O91D |
| IARC | 3 (Vol. 30, Sup 7) 1987 |
| NIST Chemistry Reference | Phosphorothioic acid, o,o-dimethyl-o-p-nitrophenyl ester(298-00-0) |
| Pesticides Freedom of Information Act (FOIA) | Methyl parathion |
| EPA Substance Registry System | Methyl parathion (298-00-0) |
Safety
| Symbol(GHS) |
  
|
|||||||||
| Signal word | Danger | |||||||||
| Hazard statements | H300+H330-H311-H373-H410 | |||||||||
| Precautionary statements | P260-P273-P280-P302+P352+P312-P304+P340+P310-P314 | |||||||||
| Hazard Codes | T+;N,N,T+,Xn,F | |||||||||
| Risk Statements | 5-24-26/28-48/22-50/53-10-67-66-51/53-36-20/22-11-65-38 | |||||||||
| Safety Statements | 28-36/37-45-60-61-26-62-16 | |||||||||
| RIDADR | UN 2052/2783/2811 | |||||||||
| WGK Germany | 3 | |||||||||
| RTECS | TG0175000 | |||||||||
| HazardClass | 6.1(a) | |||||||||
| PackingGroup | II | |||||||||
| HS Code | 29201100 | |||||||||
| Hazardous Substances Data | 298-00-0(Hazardous Substances Data) | |||||||||
| Toxicity | LD50 in male, female rats (mg/kg): 14, 24 orally; 67, 67 dermally (Gaines) | |||||||||
| NFPA 704: |
|