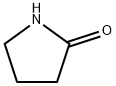
2-Pyrrolidinone occurs as a colorless or slightly grayish liquid, as white or almost white crystals, or colorless crystal needles. It has a characteristic odor. miscible with water, alcohol, ether, chloroform, benzene, ethyl acetate and carbon disulfide, insoluble in petroleum ether.
2-Pyrrolidinone is a widely used organic polar solvent for various applications. 2-Pyrrolidinone is also an intermediate in the manufacture of polymers.
2-pyrrolidone widely exists in various physiologically active natural products in nature. For example, it is the main structural unit of gonadotropin releasing hormone. At the same time, 2-pyrrolidone is an important raw material and intermediate of medicine, pesticide, dye, peptide and other chemicals. If it is used as the end chain of peptide, it also plays a stable role in the conformation of the compound. Many polysubstituted 2-pyrrolidones have been used in the synthesis and production of a variety of drugs and applied for patents.
The synthesis of 2-pyrrolidone was first reported in 1889 as the product of
dehydration of 4-aminobutanoic acid. It is produced commercially by condensation
of butyrolactone with ammonia, a method first described in 1936. Other
synthetic routes include carbon monoxide insertion into allylamine, hydrolytic
hydrogenation of succinonitrile, and hydrogenation of ammoniacal solutions of
maleic and succinnic acids (Hort and Anderson 1978).
Pyrrolidone is prepared from butyrolactone by a Reppe process, in which acetylene is reacted with formaldehyde.
ChEBI: 2-Pyrrolidinone is the simplest member of the class of pyrrolidin-2-ones, consisting of pyrrolidine in which the hydrogens at position 2 are replaced by an oxo group. The lactam arising by the formal intramolecular condensation of the amino and carboxy groups of gamma-aminobutyric acid (GABA). It has a role as a polar solvent and a metabolite.
2-Pyrrolidone undergoes the reactions of a typical lactam, e.g. ring opening, attack on the carbonyl group, and replacement of hydrogens alpha to the carbonyl group. Strong acids and bases catalyze the hydrolysis of 2-pyrrolidone to 4-aminobutanoic acid (GABA). The hydrogen atom on the nitrogen atom is easily replaced by alkylation reactions with alkyl halide or sulfates, or reaction with acid anhydrides, acyl halides, ethylene oxide, and styrene. Condensation reactions with secondary amines and alcohols, and O-alkylation reactions occur at the carbonyl group. In the presence of anionic catalyst systems, 2-pyrrolidone is polymerized to polypyrrolidone, nylon-4 (Hort and Anderson 1978).
Exposure to 2-pyrrolidone produces irritation to the eyes, mucous membranes, and
skin. Although reported to be a skin sensitizer in animal tests, there is no indication
that 2-pyrrolidone is a skin sensitizer in human exposures (Anon 1975). 2-Pyrrolidone
has been reported to enhance the permeability of human skin for methanol,
but reduced the permeability for octanol (Southwell et al 1983).
Flammability and Explosibility
Non flammable
Pharmaceutical Applications
Pyrrolidone and N-methylpyrrolidone are mainly
used as solvents in veterinary injections. Pyrrolidone has been
shown to be a better solubilizer than glycerin, propylene glycol, or
ethanol. They have also been suggested for use in human
pharmaceutical formulations as solvents in parenteral, oral, and
topical applications. In topical applications, pyrrolidones appear to
be effective penetration enhancers. Pyrrolidones have also been
investigated for their application in controlled-release depot
formulations.
2-Pyrrolidone is used as an intermediate for synthesis of l-vinyl-2-pyrrolidone and
various TV-methylol derivatives used as textile-finishing agents; as a solvent for
various polymers, chlordane and DDT, d-sorbitol, glycerin, and sugars; and as a
decolorizing agent for kerosene, fatty oils, and rosins. N-methyl-2-pyrrolidone and
2-pyrrolidone are utilized in petroleum refining to selectively extract aromatics
from paraffinic hydrocarbons. 2-Pyrrolidone is used as a plasticizer and coalescing
agent for acrylic latices and acrylic/styrene copolymers in emulsion coatings, i.e.
floor waxes. A linear high molecular weight polyamide polymer of 2-pyrrolidone,
nylon-4, is used as a textile fiber, injection molding compound, and film-forming
polymer (Anon. 1975; Hort and Anderson 1978).
Pyrrolidones are mainly used in veterinary injections and have also
been suggested for use in human oral, topical, and parenteral
pharmaceutical formulations. In mammalian species, pyrrolidones
are biotransformed to polar metabolites that are excreted via the
urine. Pyrrolidone is mildly toxic by ingestion and subcutaneous
routes; mutagenicity data have been reported.
LD50 (guinea pig, oral): 6.5 g/kg
LD50 (rat, oral): 6.5 g/kg
A metabolite of 2-pyrrolidone, 4-aminobutanoic acid has been identified in
animals (Lundgren et al 1980). 2-Pyrrolidone has been reported to be an endogenous
constituent in the brains of mice (Callery et al 1978) and bovine (Mori et al
1975). The aliphatic polyamine putrescine has been demonstrated to be metabolized
to 2-pyrrolidone in rat liver slices (Lundgren and Hankins 1978; Lundgren et
al 1985) and to lesser extent by slices of spleen and lung, but not in tissue slices
from kidney, brain, heart, or rear leg muscle (Lundgren and Hankins 1978). The
metabolism of putrescine is catalyzed by the microsomal enzyme diamine oxidase
(EC 1.4.3.6) to 4-aminobutyraldehyde, which is subsequently oxidized to the
neurotransmitter 4-aminobutanoic acid (4-aminobutyric acid, GAB A) or is cyclized
to delta1-pyrroline (Seiler 1980; Lundgren et al 1980; Callery et al 1980),
which is in turn oxidized to 5-hydroxy-2-pyrrolidone (Lundgren and Fales 1980).
There is evidence that 5-hydroxy-2-pyrrolidone is further metabolized to succinimide,
malimide, 2- and 3-hydroxysuccinamic acids, maleamic acid, and carbon
dioxide (Bandle et al 1984). An enzyme system residing in the soluble fraction of
rabbit liver catalyzes the conversion of delta'-pyrroline to ?-aminobutyric acid and
its lactam, 2-pyrrolidone (Callery et al 1982). 2-Pyrrolidone has been identified as a urinary metabolite of N-nitrosopyrrolidine (Cottrell et al 1980) and the drug
methadone (Kreek 1980).
Pyrrolidone is chemically stable and, if it is kept in unopened
original containers, the shelf-life is approximately one year.
Pyrrolidone should be stored in a well-closed container protected
from light and oxidation, at temperatures below 20°C.
Pyrrolidone is incompatible with oxidizing agents and strong acids.