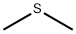Dimethyl sulfide, a sulfur ether compound, shares structural and property similarities with dimethyl ether. It is a colorless, volatile liquid that exhibits a distinct seafood-like odor. This compound is naturally generated through protein decomposition and serves as one of the culprits responsible for the fishy scent often associated with seafood.
Methyl sulfide, also known as Dimethyl sulfide, is a clear colorless volatile liquid that has an intense, unpleasant odor, wild radish, cabbage-like. It can be tolerated as a green vegetable note only at very low levels (0.1 - 3.0 ppm). Dimethyl sulfide is soluble in ethanol and ether, but insoluble in water.
Reported found in American peppermint oil, the oil of Algerian geranium, mint fractions, butter, orange,
orange juice and grapefruit juice, black currant, berries, asparagus, kohlrabi, cabbage, carrot, celery, onion, garlic, peas,
potato, rutabaga, sauerkraut, tomato, Scotch spearmint oil, parsley, wheat bread, many cheeses, yogurt, milk, cream, but�termilk, raw and cooked egg, fish, chicken, cooked beef, mutton, pork liver, hop oil, beer, cognac rum, grape wines, sherry,
tea, roasted filberts and peanuts, oats, soybean, olive, beans, mushroom, starfruit, trassi, Bantu beer, macadamia nut, mango,
cauliflower, Brussels sprouts, rice, sake, buckwheat, sweet corn, malt, wort, dried bonito, krill, shrimp, oysters, truffle, okra,
crab, clam and scallop
Dimethyl sulfide undergoes thiolation reaction with hydrogen sulfide (H2S) in the presence of tungsten-zirconia (WO3/ZrO2) catalyst to form methanethiol.
Dimethyl sulfide is used as a displaceable ligand in chloro(dimethyl sulfide)gold(I) and other coordination compounds, as a reducing agent in ozonolysis reactions as well as a food flavoring component. Further, it is utilized for the preparation of dimethyl sulfoxide, which is a common and important industrial solvent. It finds application in the petroleum industry.
By reaction of potassium sulfide with methyl chloride in methanol solution; from potassium methyl sulfate and potas�sium sulfide
ChEBI: Dimethyl sulfide is a methyl sulfide in which the sulfur atom is substituted by two methyl groups. It is produced naturally by some marine algae. It has a role as a bacterial xenobiotic metabolite, a marine metabolite, an EC 3.5.1.4 (amidase) inhibitor, an algal metabolite and an Escherichia coli metabolite.
Detection: 0.3 to 10 ppb. Aroma characteristics at 0.5%: sulfureous, dimethyl sulfide, creamy, tomato,
fishy, scallop, berry fruity and vegetative nuances
Taste characteristics at 1 to 5 ppm: sulfureous, vegetative tomato, sweet, creamed corn, corn and asparagus
with a dairy creaminess and a slight minty afternote, alliaceous.
A clear colorless to straw colored liquid with a disagreeable odor. Flash point less than 0°F. Less dense than water and slightly soluble in water. Vapors are heavier than air.
Highly flammable. Slightly soluble in water.
Organosulfides, such as Dimethyl sulfide, are incompatible with acids, diazo and azo compounds, halocarbons, isocyanates, aldehydes, alkali metals, nitrides, hydrides, and other strong reducing agents. Reactions with these materials generate heat and in many cases hydrogen gas. Many of these compounds may liberate hydrogen sulfide upon decomposition or reaction with an acid. Dimethyl sulfide rapidly decomposes dibenzoyl peroxide explosively in the absence of solvent, [J. Org. Chem., 1972, 37, 2885]. The sulfide also decomposes xenon difluoride explosively at ambient temps, [J, Chem Soc., 1984, 2827]. Interaction of Dimethyl sulfide and oxygen is explosive at 210°C and above, [Atmos. Environ., 1967, 1, 491-497]. A delayed explosion occurred in a system containing nitric acid, Dimethyl sulfide, and 1, 4-dioxane, even with cooling with liquid nitrogen, [Chem. Abs., 1972, 76, 13515].
Inhalation causes moderate irritation of upper respiratory system. Contact of liquid with eyes causes moderate irritation. Repeated contact with skin may extract oils and result in irritation. Ingestion causes nausea and irritation of mouth and stomach.
Poison by inhalation.
Moderately toxic by ingestion. A skin and
severe eye irritant. A very dangerous fire
hazard when exposed to heat or flame.
Explosive in the form of vapor when
exposed to heat or flame. Can react
vigorously with oxidizing materials. To fight
fire, use CO2, dry chemical. When heated to
decomposition it emits highly toxic fumes of
SOx and may explode. See also SULFIDES.
It is used as a presulfiding agent forvariety of catalysts used by petroleum industry; a gas odorant; catalyst impregnator; as a solvent for anhydrous mineral salts; a chemical intermediate for solvents and dimethylsulfoxide; as a flavoring ingredient in foods and beve
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with dimethyl sulfide you should betrained on its proper handling and storage. Before enteringconfined space where this chemical may be present, checkto make sure that an explosive concentration does not exist.Store in tightly closed containers in a cool, well-ventilatedarea. Metal containers involving the transfer of this chemical should be grounded and bonded. Where possible, automatically pump liquid from drums or other storagecontainers to process containers. Drums must be equippedwith self-closing valves, pressure vacuum bungs, and flamearresters. Use only nonsparking tools and equipment, especially when opening and closing containers of this chemical. Sources of ignition, such as smoking and open flames,are prohibited where this chemical is used, handled, orstored in a manner that could create a potential fire orexplosion hazard. Wherever this chemical is used, handled,manufactured, or stored, use explosion-proof electricalequipment and fittings.
This compound requires a shipping label of“FLAMMABLE LIQUID.” It falls in Hazard Class 3 andPacking Group II.
Purify dimethyl sulfide via the Hg(II) chloride complex by dissolving 1 mole of Hg(II)Cl2 in 1250mL of EtOH and slowly adding the boiling alcoholic solution of Me2S to give the right ratio for 2(CH3)2S.3HgCl2. After recrystallisation of the complex to constant melting point, 500g of complex is heated with 250mL conc HCl in 750mL of water. The sulfide is separated, washed with water, and dried with CaCl2 and CaSO4. Finally, it is distilled under reduced pressure from sodium. Precautions should be taken (efficient fume hood) because of its very UNPLEASANT ODOUR.[Beilstein 1 IV 1275.]
Reacts violently with strong ox