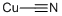Copper(I) cyanide, CuCN, [544-92-3], MW 89.56, MP 474°C, d 2.92, is white when pure, but usually available as an off-white or cream-colored powder and is insoluble in water and dilute acids but dissolves in complexing media such as ammonia and alkali cyanide solutions. It is produced by the reaction of sodium cyanide with copper(I) chloride solutions or by the reaction of copper(II) sulfate solutions with alkali cyanide and sodium hydrogen sulfite. It is used extensively in the electroplating industry and as a polymerization catalyst in organic reactions.
Copper(I) cyanide is used in copper plating of nickel, chromium, zinc alloys,
steel, and other metals or alloys. Such copper plating imparts brightness,
smoothness, hardness, and strength. The cyanide solution employed for copper electroplating consists of copper cyanide and sodium cyanide. Other applications of this compound are as an insecticide, a catalyst in polmerization, and
as an antifouling agent in marine paints.
Copper(I) cyanide is a precipitate obtained by adding potassium cyanide solution to an aqueous solution of Cu2+ salt:
COPPER(I) CYANIDE 2652CuCl2 + 4KCN → 2CuCN + C2N2 + 4KCl
The Cu2+ to CN¯ molar ratio should be 1:2. The precipitate dissolves in an excess of cyanide, forming soluble ions Cu(CN)2¯ , Cu(CN)32¯, and Cu(CN)43¯.
off-white to green powder. Insoluble in water,soluble in HCI, Nl40H, and potassium cyanide. Used in Sandmeyer's reaction to synthesize aryl cyanides. Toxic by skin absorption, through open wounds, by ingestion, and by inhalation of hydrogen cyanide that arises from slight decomposition. Produces toxic oxides of nitrogen in fires.
Cuprous cyanide is a white crystalline substance.
Cream-colored powder or green orthorhombic or red monoclinic crystals; density 2.90 g/cm3; melts at 474°C; decomposes at higher temperatures; practically insoluble in water, ethanol, and cold dilute acids; dissolves in ammonium hydroxide and potassium cyanide solutions.
Cuprous cyanide is used in electroplating; as an insecticide and fungicide; and as a catalyst for polymerization.
Copper(I) cyanide is commonly used as a polymerization catalyst. The compound is useful in organic synthesis, as a catalyst, reagent in the preparation of nitriles(sandmeyer reaction). It is also used in electroplating copper and iron, silver plating, brass plating, and copper-tin alloy plating. It is used to make phthalocyanine dyes and pigments.
In electroplating Cu or Fe; as insecticide, fungicide; as antifouling agent in marine paints; as polymerization catalyst.
Copper(I) Cyanide is decomposed by acids to give off hydrogen cyanide, a flammable poisonous gas. Tends to explosive instability. Capable of violent oxidation under certain condition: fusion with metal chlorates, perchlorates, nitrates or nitrites can cause explosions [Bretherick, 1979 p. 101]. Reacts with incandescence with magnesium [Mellor, 1940, Vol. 4, 271].
Cuprous cyanide is a highly toxic substance. The toxic routes are inhalation of dust, ingestion, and skin contact. Toxicology and LD50 values for this compound are not reported. Because it is slightly soluble in water, its dissociation to cuprous and cyanide ions in the body may not be significant. The role of cyanide ion in the toxicity of cuprous cyanide is not established. The inhalation hazard, however, is attributable to copper. It is a skin irritant.
Special Hazards of Combustion Products: Toxic hydrogen cyanide gas may form in fires.
Flammability and Explosibility
Not classified
A poison. Reacts
violently with magnesium. When heated to
decomposition it emits very toxic CNand
NOx. See also CYANIDE and COPPER
COMPOUNDS.
Copper cyanide is used in electroplating copper on iron; and as an insecticide and a catalyst.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least30 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit. Speedin removing material from skin is of extreme importance.Remove and isolate contaminated clothing and shoes at thesite. Keep victim quiet and maintain normal body temperature. Effects may be delayed. Keep victim underobservation.
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Prior to working with thischemical you should be trained on its proper handling andstorage. Store in tightly closed containers in a cool, wellventilated area away from strong oxidizers.
UN1587 Copper cyanide, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Wash the cyanide thoroughly with boiling H2O, then with EtOH. Dry it at 100o to a fine soft powder. It dissolves in excess alkali cyanide solutions to form the very soluble complex ion Cu(CN)43-. [Bassett & Corbett J Chem Soc 125 1660 1924, Barber J Chem Soc 79 1943.]
Contact with heat, strong acids (HCl, H2SO4, HNO3) forms deadly hydrogen cyanide gas. May release hydrogen cyanide on contact with moisture. Incompatible with strong oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, acetylene gas, and chemically active metals, such as potassium, sodium, magnesium, and zinc.
Copper-containing soluble wastes can be concentrated through the use of ion exchange, reverse osmosis, or evaporators to the point where copper can be electrolytically removed and sent to a reclaiming firm. If recovery is not feasible, the copper can be precipitated through the use of caustics and the sludge deposited in a chemical waste landfill. Copper-containing wastes can be concentrated to the point where copper can be electrolytically removed and reclaimed. If recovery is not feasible, the copper can be precipitated by alkali; the cyanide destroyed by alkaline oxidation yielding a sludge which can be sent to a chemical waste landfill. In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.