Chlorobenzene is a monocyclic aromatic compound with one hydrogen atom on the benzene ring substituted with one chlorine. It is produced by chlorination of benzene in the liquid phase with a catalyst. Chlorobenzene is a colourless, flammable liquid with a sweet almond-like odor, at ambient temperature with a relatively high vapour pressure, moderate octanol-water partition coefficient (log 2.8) and moderate to low water solubility (497.9 mg/L @ 25℃). Chlorobenzene has a high solubility in nonpolar solvents, however, it is almost insoluble in water. Technical grade Chlorobenzene is typically 99% pure with < 0.05% benzene and < 0.1% dichlorobenzenes as contaminants. It is a common solvent and a widely used intermediate in the manufacture of other chemicals. Rhodococcus phenolicus is a bacterium species able to degrade chlorobenzene as sole carbon sources.
- Chlorobenzene is used primarily as raw material for the synthesis of o- and p-nitrochlorobenzene and 2,4-dinitrochlorobenzene.Important quantitative chemical conversions other than the production of nitrochlorobenzenes are the production of diphenyl oxide and diphenyldichlorosilane.
- Chlorobenzene is mainly used as raw material for the synthesis of chemicals including triphenylphosphine (catalyst for organic synthesis), phenylsilane, and thiophenol (pesticide and pharmaceutical intermediate). It is also used as raw material for the synthesis of solvent for organic synthesis reactions including methylenediphenyldiisocyanate, urethane raw material, agricultural adjuvants, paint and ink, and cleaning solvent for electronics.
- manufacture of phenol, aniline, DDT; solvent for paints; heat transfer medium.
- Chlorobenzene is used as a process solvent in the production of isocyanates such as MDI and TDI and as a solvent in various crop protection formulations. It is further used as a solvent in condensation reactions in the dyes industry.
- Chlorobenzene is a basic substance used in chemical syntheses with 95% of the quantity used converted in closed systems to intermediate and final products.
- It is used as a process solvent in the manufacture of three indigoid dyes and pigments. All the pigments and dyes are thioindigoid colors.
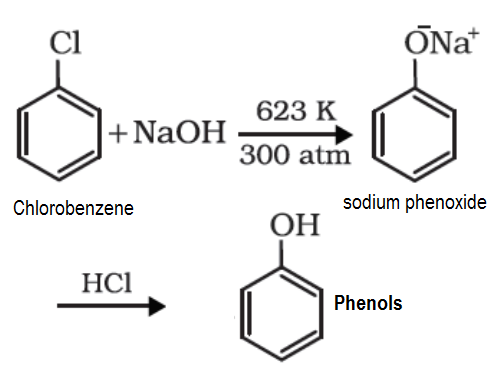
- Chlorobenzene is an example of haloarenes which is formed by mono substitution of benzene ring. When chlorobenzene is fused with sodium hydroxide at 623K and 320 atm sodium phenoxide is produced. Finally, sodium phenoxide on acidification gives phenols.
The toxic effects of chlorobenzene on humans were exhaustion, nausea, lethargy, headache and irritation to the upper respiratory tract and eye. Contact of chlorobenzene with the skin induced irritation. No reports were obtained on sensitization by chlorobenzene in this investigation.
The oral LD50 values of chlorobenzene were 1,445 mg/kg in mice, 1,427 to 3,400 mg/kg in rats and 2,250 to 2,830 mg/kg in rabbits. The LC50s following 6-hr inhalation exposure were 1,889 ppm in mice and 2,968 ppm in rats.
Chlorobenzene is a colorless liquid with analmond-like odor. The odor threshold is between 0.1 and3.0 μg/L. Also reported in the literature at 0.68 and0.741 ppm. Molecular weight=112.56; Boilingpoint=131-132℃; Freezing/Melting point=2 45℃;Flash point=28℃; Autoignition temperature=593℃.Flammable limits are: LEL=1.3%; UEL=9.6%. HazardIdentification (based on NFPA 704 M Rating System):Health 2, Flammability 3, Reactivity 1. Insoluble in water.
Chlorobenzene, also known as monochlorobenzene or MCB, is a colourless flammable liquid with an aromatic almond-like odor, Chlorination of benzene in the presence of a catalyst (FeCl3 or AICI3) yields chlorobenzene as the first product. It is insoluble in water and miscible with organic solvents. Chlorobenzene has a good solvency for fats, oils, resins, polymers, binders, rubber, and chlorinated rubber. Cellulose ethers dissolve in the presence of small amounts of alcohols; cellulose nitrate is insoluble. Chlorobenzene is a solvent in the production of bitumen and asphalt coatings for building protection.
Clear, colorless, flammable liquid with a sweet almond, medicinal or mothball-like odor. An odor threshold concentration of 210 ppbv was reported by Leonardos et al. (1969). At 40 °C, the lowest concentration at which an odor was detected was 190 μg/L. At 25 °C, the lowest concentration at which a taste was detected was 190 μg/L (Young et al., 1996). The average least detectable odor threshold concentration in water at 60 °C was 0.08 mg/L (Alexander et al., 1982). Cometto-Muiz and Cain (1994) reported an average nasal pungency threshold concentration of 10,553 ppmv. Chlorobenzene can evaporate when exposed to air. It dissolves slightly when mixed with water. It is moderately soluble in water; up to 1,000 milligrams will mix with a liter of water. Chlorobenzene is slightly persistent in water, with a half-life of between 2 to 20 days. It persists in soil (several months), in air (3.5 days), and water (less than 1 day).
Chlorobenzene is a halogenated benzene that is used as a solvent for paints, as a heat transfer medium, and in the manufacture of phenol, aniline and nitrochlorobenzenes. Now chlorobenzene is mainly used as a solvent for pesticide formulations, diisocyanate manufacture, degreasing automobile parts, and for the production of nitrochlorobenzene and chemical toxicity QSAR research for agricultural pollution.
Chlorobenzene is produced by chlorination of benzene in the presence of a catalyst, and is produced as an end product in the reductive chlorination of di- and trichlorobenzenes.
ChEBI: Chlorobenzene is the simplest member of the class of monochlorobenzenes, that is benzene in which a single hydrogen has been substituted by a chlorine. It has a role as a solvent.
Chlorobenzene is a colorless to clear, yellowish liquid with a sweet almond-like odor. Flash point 84°F. Practically insoluble in water and somewhat denser than water (9.2 lb / gal). Vapors heavier than air. It can be converted to phenol by reaction with sodium hydroxide under extreme conditions (300°C and 200 atmospheres pressure). Used to make pesticides, dyes, and other chemicals.
Highly flammable. Insoluble in water.
Chlorobenzene undergoes a sometimes explosive reaction with powdered sodium or phosphorus trichloride + sodium. May react violently with dimethyl sulfoxide. Reacts vigorously with oxidizing agents. Attacks some forms of plastic, rubber and coatings. Forms a shock sensitive solvated salt with silver perchlorate.
A possible carcinogen. Avoid inhalation
and skin contact. Moderate fire risk. Explosive limits 1.8–9.6%.
Irritating to skin, eyes and mucous membranes. Repeated exposure of skin may cause dermatitis due to defatting action. Chronic inhalation of vapors or mist may result in damage to lungs, liver, and kidneys. Acute vapor exposures can cause symptoms ranging from coughing to transient anesthesia and central nervous system depression.
Limited information is available on the acute (short-term) effects of chlorobenzene. Acute inhalation exposure of animals to chlorobenzene produced narcosis, restlessness, tremors, and muscle spasms. Chronic (long-term) exposure of humans to chlorobenzene affects the central nervous system (CNS). Signs of neurotoxicity in humans include numbness, cyanosis, hyperesthesia (increased sensation), and muscle spasms. No information is available on the carcinogenic effects of chlorobenzene in humans. EPA has classified chlorobenzene as a Group D, not classifiable as to human carcinogenicity.
Flammable liquid; flash point (closed cup) 29°C (84°F); vapor pressure 8.8 torr at 20°C (68°F); autoignition temperature 638°C (1180°F). When heated to decomposition this compound emits toxic fumes of hydrogen chloride gas, CO and CO2.
Chlorobenzene vapors form explosive mixtures with air within the range 1.3-7.1% by volume in air. It is incompatible with strong oxidizing agents and dimethyl sulfoxide. Dimethyl sulfoxide decom poses violently in contact with chloroben zene (NFPA 1997). Many metal perchlorates, such as those of silver and mercury, may form shock-sensitive solvated perchlorates that may explode on impact.
Flammability and Explosibility
Flammable
Suspected carcinogen.
Moderately toxic by ingestion and
intraperitoneal routes. Experimental
teratogenic and reproductive effects.
Mutation data reported. Strong narcotic with
slight irritant qualities. Dichlorobenzols are
strongly narcotic. Little is known of the
effects of repeated exposures at lower
concentrations, but it may cause hdney and
liver damage. The industrial illnesses
reported may possibly be due to
nitrobenzol. Dangerous fire hazard when
exposed to heat or flame. Moderate
explosion hazard when exposed to heat or
flame. Potentially explosive reaction with
powdered sodium or phosphorus trichloride
+ sodtum. Violent reaction with AgClO4.
Reacts vigorously with oxidizers. See also
CHLORINATED HYDROCARBONS,
AROMATIC. To fight fire, use foam, CO2,
dry chemical, water to blanket fire.
Associated with EPA Superfund sites
Chlorobenzene is used in the manufacture of aniline, phenol, and chloronitrobenzene; as an intermediate in the manufacture of dyestuffs and many
pesticides, as a solvent; and emulsifier.
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.Medical observation is recommended.Note to physician: Treat for methemoglobinemia.Spectrophotometry may be required for precise determination of levels of methemoglobinemia in urine.
Chlorobenzene was not mutagenic in a
variety of bacterial and yeast assays. Existing
data suggest that genotoxicity may not be an
area of concern for chlorobenzene exposure in
humans.
Chlorobenzene's production and use as a chemical intermediate, solvent, and heat transfer medium may result in its release to the environment through various waste streams. If released to air, chlorobenzene will exist solely as a vapor in the atmosphere. Photochemically produced hydroxyl radicals will ultimately degrade vapor-phase chlorobenzene in less than 24h.
Biological. In activated sludge, 31.5% of the applied chlorobenzene mineralized to carbon dioxide after 5 d (Freitag et al., 1985). A mixed culture of soil bacteria or a Pseudomonas sp. transformed chlorobenzene to chlorophenol (Ballschiter and Scholz, 1980). Pure microbial cultures isolated from soil hydroxylated chlorobenzene to 2- and 4-chlorophenol (Smith and Rosazza, 1974). Chlorobenzene was statically incubated in the dark at 25 °C with yeast extract and settled domestic wastewater inoculum. At a concentration of 5 mg/L, biodegradation yields at the end of 1 and 2 wk were 89 and 100%, respectively. At a concentration of 10 mg/L, significant degradation with gradual adaptation was observed.Complete degradation was not observed until after the 3rd week of incubation (Tabak et al.,1981).
https://www.epa.gov
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with chlorobenzene you should be trainedon its proper handling and storage. Before entering confinedspace where this chemical may be present, check to makesure that an explosive concentration does not exist.Chlorobenzene must be stored to avoid contact with strongoxidizers (such as chlorine, bromine, and fluorine), sinceviolent reactions occur. Store in tightly closed containers ina cool, well-ventilated area away from heat, sparks, orflames. Sources of ignition, such as smoking and openflames are prohibited where Chlorobenzene is used, handled, or stored in a manner that could create a potential fireor explosion hazard. Metal containers involving the transferof=gallons or more of Chlorobenzene should be groundedand bonded. Drums must be equipped with self-closingvalves, pressure vacuum bungs, and flame arresters. Useonly nonsparking tools and equipment, especially whenopening and closing containers of Chlorobenzene.
UN1134 Chlorobenzene, Hazard Class: 3;
Labels: 3-Flammable liquid.
The main impurities are likely to be chlorinated impurities originally present in the *benzene used in the synthesis of chlorobenzene, and also unchlorinated hydrocarbons. A common purification procedure is to wash it several times with conc H2SO4 then with aqueous NaHCO3 or Na2CO3, and water, followed by drying with CaCl2, K2CO3 or CaSO4, then with P2O5, and distilling. It can also be dried with Linde 4A molecular sieve. Passage through, and storage over, activated alumina has been used to obtain low conductance material. [Flaherty & Stern J Am Chem Soc 80 1034 1958, Beilstein 5 H 199, 5 IV 640.]
In the ambient atmosphere, chlorobenzene will exist as
a vapor, and will be degraded by reaction with photochemically
produced hydroxyl radicals, with an estimated half-life of 21 days. It can be removed from the air by rain. Photolysis halflives
of 4–18 h were measured in aqueous media. If released to
soil, chlorobenzene is expected to have very high to moderate
mobility based on a Koc range of 4.8–313. Moist soil surfaces
will favor volatilization based upon Henry’s Law constant of
3.11×103 atm-cu m mol-1. Chlorobenzene may volatilize
from dry soil surfaces as well. If released into water, chlorobenzene
may adsorb to suspended solids and sediment based
on the Koc values. Volatilization from water surfaces is expected
to be an important fate process based on this compound’s
Henry’s Law constant. Estimated volatilization half-lives for
a model river and model lake are 3.4 h and 4.3 days, respectively.
Reported bioconcentration in aquatic organisms is low
to high, provided the compound is not metabolized by the
organism. Hydrolysis is not expected to be an important
environmental fate process since this compound lacks functional
groups that hydrolyze under environmental conditions.
Biodegradation results are variable based on soil type and
microbial diversity. In river water, the biodegradation half-life
was reported to be 150 and 75 days in the sediment.
Reacts violently with strong oxidizers;
dimethyl sulfoxide; sodium powder; silver perchlorate;
causing fire and explosion hazard. Attacks some plastics,
rubber, and coatings. Decomposes on heating, producing
phosgene and hydrogen chloride fumes.
Incineration, preferably after
mixing with another combustible fuel; care must be exercised to assure complete combustion to prevent the formation of phosgene; an acid scrubber is necessary to remove
the halo acids produced.